Percutaneous balloon valvuloplasty is a technique used since the late 1980s to treat congenital aortic stenosis.1 Published studies, either on this technique alone or comparing it to surgical valvotomy, have identified predictors of unfavourable outcomes, such as severe thickening of the valve, unicuspid morphology, neonatal critical aortic stenosis or a balloon-to-annulus ratio greater than 1. There have been no statistically significant differences in survival, residual regurgitation or the need of valve replacement between the 2 techniques. The only aspect that may favour the use of the open surgery is the need of reintervention. Due to the reduced invasiveness and comparable outcomes of percutaneous balloon valvuloplasty, this technique is currently considered the first-line treatment for congenital aortic stenosis.2,3
We present a retrospective analysis of all the patients with congenital aortic stenosis that have undergone percutaneous balloon valvuloplasty in our hospital since 1990. The sample included 45 patients with a mean age of 4.1 ± 5.7 years, with a predominance of the male sex (57.8%); 28 patients (62.2%) were aged less than 1 year and 9 less than 1 month. The valvular morphology was bicuspid in most cases (80%). Six of the patients (13.3%) had previously undergone surgical valvotomy, as they were initially not considered good candidates for the percutaneous approach because their aortic valves were severely dysplastic.
The procedure was considered indicated for patients who were symptomatic or with a peak gradient greater than 50 mm Hg. The intervention was elective in most patients, but out of 8 who had critical stenosis, 2 developed cardiogenic shock and require urgent intervention.
All the interventions were performed under general anaesthesia with a femoral arterial approach with a 4 to 8 F catheter. Catheters were chosen to achieve a ratio of approximately 1:1 (0.90–1.1) relative to the annulus (measured by both ultrasound and angiography). We considered the intervention effective if it achieved a reduction of the peak gradient to less than 35 mm Hg with residual regurgitation grade 1 or less, which was accomplished in 84.1% of cases (Table 1).
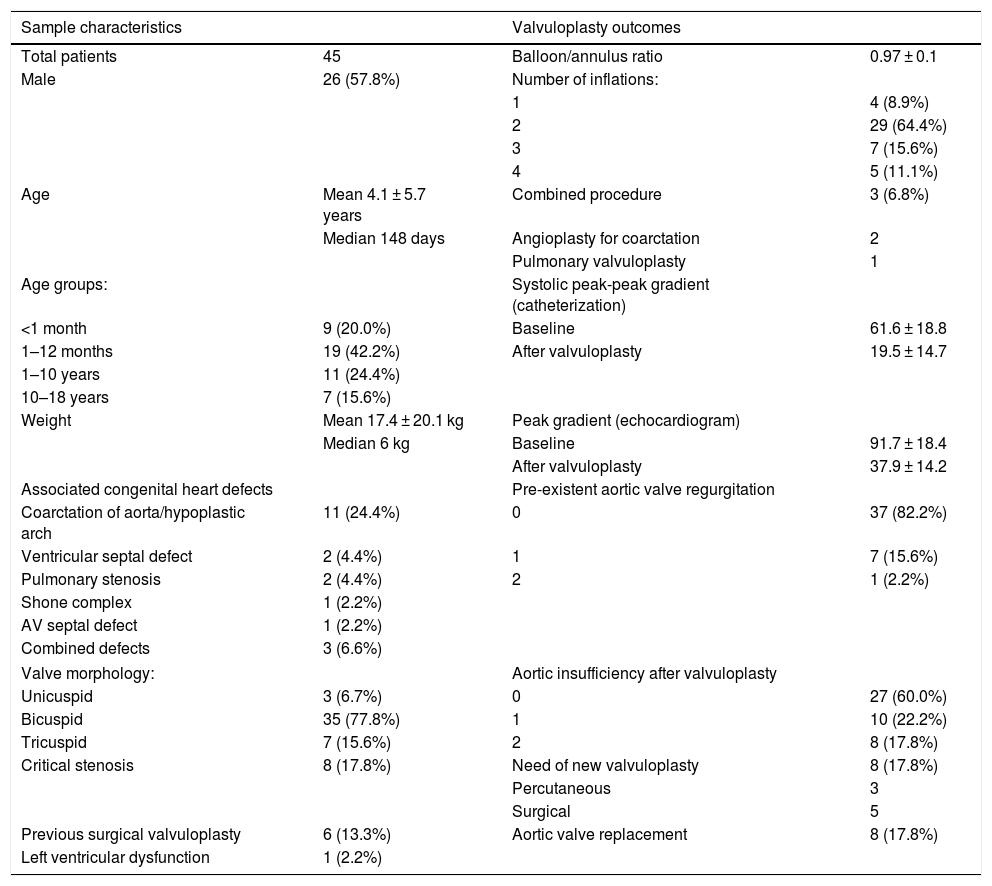
Demographic characteristics of the sample and outcomes of the procedure.
| Sample characteristics | Valvuloplasty outcomes | ||
|---|---|---|---|
| Total patients | 45 | Balloon/annulus ratio | 0.97 ± 0.1 |
| Male | 26 (57.8%) | Number of inflations: | |
| 1 | 4 (8.9%) | ||
| 2 | 29 (64.4%) | ||
| 3 | 7 (15.6%) | ||
| 4 | 5 (11.1%) | ||
| Age | Mean 4.1 ± 5.7 years | Combined procedure | 3 (6.8%) |
| Median 148 days | Angioplasty for coarctation | 2 | |
| Pulmonary valvuloplasty | 1 | ||
| Age groups: | Systolic peak-peak gradient (catheterization) | ||
| <1 month | 9 (20.0%) | Baseline | 61.6 ± 18.8 |
| 1–12 months | 19 (42.2%) | After valvuloplasty | 19.5 ± 14.7 |
| 1–10 years | 11 (24.4%) | ||
| 10–18 years | 7 (15.6%) | ||
| Weight | Mean 17.4 ± 20.1 kg | Peak gradient (echocardiogram) | |
| Median 6 kg | Baseline | 91.7 ± 18.4 | |
| After valvuloplasty | 37.9 ± 14.2 | ||
| Associated congenital heart defects | Pre-existent aortic valve regurgitation | ||
| Coarctation of aorta/hypoplastic arch | 11 (24.4%) | 0 | 37 (82.2%) |
| Ventricular septal defect | 2 (4.4%) | 1 | 7 (15.6%) |
| Pulmonary stenosis | 2 (4.4%) | 2 | 1 (2.2%) |
| Shone complex | 1 (2.2%) | ||
| AV septal defect | 1 (2.2%) | ||
| Combined defects | 3 (6.6%) | ||
| Valve morphology: | Aortic insufficiency after valvuloplasty | ||
| Unicuspid | 3 (6.7%) | 0 | 27 (60.0%) |
| Bicuspid | 35 (77.8%) | 1 | 10 (22.2%) |
| Tricuspid | 7 (15.6%) | 2 | 8 (17.8%) |
| Critical stenosis | 8 (17.8%) | Need of new valvuloplasty | 8 (17.8%) |
| Percutaneous | 3 | ||
| Surgical | 5 | ||
| Previous surgical valvuloplasty | 6 (13.3%) | Aortic valve replacement | 8 (17.8%) |
| Left ventricular dysfunction | 1 (2.2%) | ||
Rapid ventricular pacing was used in 70.4% of the patients (in the left ventricle with a 0.014 Biotronik Vision Wire guidewire in neonates and infants and in the right ventricle with an electrode catheter in all other patients) to improve balloon stability during inflations, which results in a statistically significant increase in the effectiveness of the procedure and reduced damage to the valve and, therefore, a lesser degree of residual regurgitation after the procedure.
The salient complications were 2 cases of femoral artery thrombosis, both in neonates, that resolved with administration of heparin, and there were no other severe complications or any deaths related to the procedure.
All patients remained in follow-up with clinical and echocardiographic assessments for a mean of 11 ± 9.8 years, and 12 of them (26.7%) required reintervention (another valvuloplasty or valve replacement). Eight patients (17.8%) required a new valvuloplasty, percutaneous in 3 cases (after a mean of 259 days) and surgical in 5 (after a mean of 2.6 years); 2 of the patients that required reintervention with surgical valvulotomy underwent yet another percutaneous balloon valvuloplasty during the follow-up.
Eight patients (18.2% of the total) required aortic valve replacement surgery during the follow-up a mean of 15.25 ± 7.8 years after the initial valvuloplasty, and 4 of them had required a second valvuloplasty in between. The survival free of aortic valve replacement was 94% at 10 years, 82% at 15 years and 58% at 20 years.
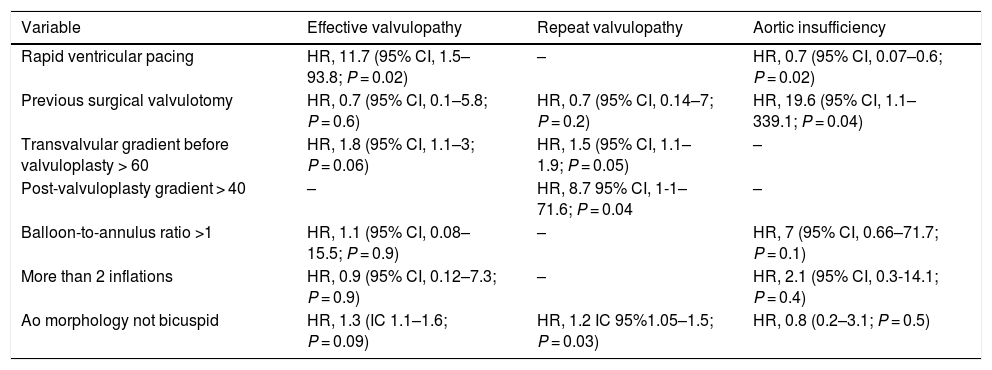
Table 2 presents the variables that were associated with effective valvuloplasty, the need of reintervention or increase grades of residual aortic regurgitation.
Variables associated with effective valvulopathy, reintervention and residual aortic regurgitation.
| Variable | Effective valvulopathy | Repeat valvulopathy | Aortic insufficiency |
|---|---|---|---|
| Rapid ventricular pacing | HR, 11.7 (95% CI, 1.5–93.8; P = 0.02) | – | HR, 0.7 (95% CI, 0.07–0.6; P = 0.02) |
| Previous surgical valvulotomy | HR, 0.7 (95% CI, 0.1–5.8; P = 0.6) | HR, 0.7 (95% CI, 0.14–7; P = 0.2) | HR, 19.6 (95% CI, 1.1–339.1; P = 0.04) |
| Transvalvular gradient before valvuloplasty > 60 | HR, 1.8 (95% CI, 1.1–3; P = 0.06) | HR, 1.5 (95% CI, 1.1–1.9; P = 0.05) | – |
| Post-valvuloplasty gradient > 40 | – | HR, 8.7 95% CI, 1-1–71.6; P = 0.04 | – |
| Balloon-to-annulus ratio >1 | HR, 1.1 (95% CI, 0.08–15.5; P = 0.9) | – | HR, 7 (95% CI, 0.66–71.7; P = 0.1) |
| More than 2 inflations | HR, 0.9 (95% CI, 0.12–7.3; P = 0.9) | – | HR, 2.1 (95% CI, 0.3-14.1; P = 0.4) |
| Ao morphology not bicuspid | HR, 1.3 (IC 1.1–1.6; P = 0.09) | HR, 1.2 IC 95%1.05–1.5; P = 0.03) | HR, 0.8 (0.2–3.1; P = 0.5) |
Ao, aorta; CI, confidence interval; HR, hazard ratio.
Six patients (13.3%) died during follow-up, corresponding to a mortality of 2% at 1 month and 6.6% at 1 year post valvuloplasty. Fifty percent of the deaths occurred in the postoperative period following surgical repair of complex heart defects (atrioventricular septal defect with aortic arch hypoplasia and coarctation of the aorta, Shone complex treated with dilatation of the aortic arch and surgical repair of the coarctation and aortic valve and supravalvular stenosis treated with the Ross-Konno procedure).
In the subset of patients with neonatal critical aortic stenosis, we found a higher peak gradient measured by echocardiography before the procedure compared to the rest of the series (91.7 ± 18.4 vs 79.4 ± 14.4; P = 0.06), with similar gradients post valvuloplasty (40 ± 2 vs 38.6 ± 7; P = 0.08). The overall reintervention-free survival was 93% at 1 year, 78.8% at 5 years and 71% at 10 years, compared to 78% at 1, 5 and 10 years in the neonatal subset, differences that were not statistically significant and were particularly biased by first-year outcomes in neonatal patients on account of the greater complexity of disease and associated defects.
Despite the limitations of our study due to the small sample size and its retrospective design, we conclude that balloon valvuloplasty is a safe and effective procedure for treatment of congenital aortic stenosis with good outcomes in the short and medium terms, although less so in the long term on account of the need for reintervention. The main goal of the intervention is to facilitate growth and development and postpone surgical valve replacement for as long as possible, an intervention that is necessary within 20 years in nearly 40% of the patients.3–5
Please cite this article as: González LF, Mata RB, Meabe JA, García ML, Miranda JMG. Vigencia de la valvuloplastia con balón en la estenosis aórtica congénita. Experiencia desde los inicios de la técnica. An Pediatr (Barc). 2022;96:265–267.