To analyze the prevalence of use of off-label and unlicensed drugs in a paediatric intensive care unit of a University Hospital.
MethodAn observational, descriptive, prospective six week pilot study in a paediatric intensive care unit. Hospitalized patients aged between 0 and 18 years were included. Each prescribed drug was evaluated taking into account indication and condition of use, according to the information available on the summary of product characteristics established by the European Medicines Agency. A sequential algorithm was defined allowing drug classification in unlicensed, off-label or approved.
Results42 patients were included. A total of 696 prescriptions, involving 102 different drugs, were analyzed. All patients had at least one off-label prescription, and a median of 8.9 off-label prescriptions were obtained. Of the total prescriptions, 8.6% were unlicensed and 53.9% corresponded to off-label use. The main reason for off-label use was by indication, followed by age and dose. A lineal tendency between off-label drug use and patient age was observed, where off-label use increased as patient age decreased. The drugs most commonly used off-label were: atropine, etomidate, dipyrone and ranitidine, and unlicensed drugs: spironolactone, sildenafil, acetazolamide and hydrochlorothiazide.
ConclusionPaediatric intensive care units are characterized by a high ratio of off-label and unlicensed prescriptions. The scarce number of studies performed in this specific and complex sub-population added inconveniency to the current lack of data on safety and efficacy for drugs in paediatrics. Performing studies with these characteristics allowing us to document practice on paediatric drug utilization is required.
Evaluar el perfil de utilización de medicamentos en situaciones no autorizadas en una unidad de cuidados intensivos pediátricos de un hospital universitario.
MétodosSe realizó un estudio observacional descriptivo prospectivo durante 6 semanas en una unidad de cuidados intensivos pediátricos. Se incluyeron pacientes ingresados con edades entre 0-18 años. Se evaluó cada uno de los medicamentos prescritos, indicación o condición de uso, según la información reflejada en las fichas técnicas autorizadas por la Agencia Europea de Medicamentos. Se definió un algoritmo secuencial para clasificar de manera estandarizada los medicamentos según la condición de prescripción en unlicensed, off-label o aprobado.
ResultadosSe incluyeron 42 pacientes, analizándose un total de 696 prescripciones, que implicaron 102 fármacos diferentes. Todos los pacientes tuvieron al menos un tratamiento off-label. El 8,6% del total de tratamientos analizados se utilizaron en condiciones unlicensed y el 53,9% en off-label. El principal motivo de uso off-label fue por indicación, seguido de la edad y dosis. Existe una relación lineal entre frecuencia de uso de medicamentos en condiciones off-label y la edad del paciente, aumentando esta frecuencia según disminuye la edad del paciente. Los medicamentos más utilizados en condiciones off-label fueron: atropina, etomidato, metamizol y ranitidina, y en condiciones unlicensed fueron: espironolactona, sildenafilo, acetazolamida e hidroclorotiazida.
ConclusiónLa unidad de cuidados intensivos pediátricos se caracteriza por un alto ratio de medicamentos prescritos en condiciones no autorizadas. La realización de estudios de estas características permite documentar la práctica clínica respecto al uso de medicamentos en condiciones distintas a las autorizadas.
Encouraging effective and safe use of drugs in children is a current worldwide necessity. The resolution of problems associated with drug use in children has been tackled over the years. The objective is clear, to provide children with safe and effective drugs including precise and updated information. However, in spite of efforts by physicians, researchers, scientific societies and health-related policies, to date, a series of obstacles still exists, preventing an adequate development of drugs for paediatric use. These barriers are well known, and amongst them we find1,2: the cost of performing corresponding studies; the difficulty in designing trials; the time necessary for completing the contemplated study periods in protocols for children, far superior to those in adults; lengthy approval procedures; the complex and specific ethical aspects involved in all research concerning children; and lastly, obtaining consent from patients who cannot concede it themselves.
Due to the lack of safety and efficacy data, the “non-approved” use of drugs in paediatrics has always involved a risk for patients. The drugs used, following the label specifications established at the beginning of commercialization have a lower tendency towards producing adverse effects than those drugs whose use in children is not authorized or is prescribed in different conditions to those stated on the label.3–9 This was revised by the European Medicines Agency (EMA) itself, concluding that the use of “non-approved” drugs increments the incidence and severity of adverse reactions.10 Nevertheless, as the percentage of drugs without paediatric indication is so high (50–90%) and affects approximately 20% of the European Union population (around 150 million people under 18 years),2 the use of drugs in these conditions is almost obligatory. It is important that children are not denied access to clearly beneficial drugs. It is neither practical nor appropriate to restrict use only to drugs approved for this age group. Health professionals are obliged to give children the best possible treatment, which invariably implies non-authorized drug use or use in conditions different to those approved.7,11,12
The aims of the present study were to characterize unlicensed and off-label drug prescription and analyze its prevalence in children admitted to a paediatric intensive care unit (PICU) of a Spanish University Hospital.
MethodsAn observational, descriptive, prospective pilot study was performed in a paediatric intensive care unit over 6 consecutive weeks, between September and October 2011. The study was carried out in the PICU of one tertiary, general, public, university hospital in the city of Madrid, Spain. The paediatric intensive care unit is equipped with 11 beds for patients from 0–18 years of age. It is predominantly a cardiac intensive care unit due to the high prevalence of cardiovascular diseases that are attended in this unit (clinical and surgical conditions). The PICU has computerized physician order entry with pharmacist order validation and is provided with automatic dispensing cabinets.
The study was based on the collection of variables related to the patients and the prescribed drugs available on clinical records, provided by the hospital's computerized system, and from information provided by the health care team. The access to clinical data followed the protocols established at the hospital and the authors signed an undertaking to preserve confidentiality and to use the data collected solely for the purpose of scientific publication.
The study was conducted after obtaining permission from the Institutional Ethics Committee. The requirement for informed consent was waived.
The study enrolled patients aged 0–18 years admitted to the PICU from September–October 2011. Patients over 18 were excluded, as well as those without social security membership, as were age, weight and diagnosis. The use of the following drugs was also excluded: nutritional preparations, standard intravenous crystalloid solutions, oxygen and heparin used to maintain patency of intravenous lines.
A data base was designed (Microsoft Access_2007; Microsoft Corporation, Redmond, WA, USA) where variables for every hospitalized patient were collected: age, sex, type of patient (medical or surgical), anatomical system related to hospitalization, principal diagnosis and optionally secondary diagnosis, and prescribed drugs. Age classification of paediatric patients was established according to the scientific guidelines about clinical investigation of medicinal products in the paediatrics population, published by the European Medicines Agency [International Conference on Harmonisation (ICH) guidance E11].13
To calculate the total number of items prescribed, the following were defined as new prescriptions: change in drug, different route of administration, different presentation, the same drug with suspended treatment for several days and re-prescription or dose change (when changing from one dose indicated on label to a dose not indicated on label).
For every prescribed drug the ATC (World Health Organisation's Anatomical Therapeutic Chemical classification system) group was registered, according to the current Nordic Council classification,14 and the route of administration and dose was reflected in the Ministry of Health. Furthermore, for every drug included, we took into account its indication and condition of use according to summary of product characteristics (SmPC) established by the EMA. Depending on use, it was considered either unlicensed or off-label, according to consensus established in 2008 by a panel of experts.15 Unlicensed use was defined by this consensus, and supported by EMA-dependent paediatric working group, as use of a drug which has never obtained a European Marketing Authorisation as a medicinal product for human use, in either children or adults. Off-label use was defined by consensus as use of any marketed drug not detailed in the SmPC, including therapeutic indication, age group in which it is used, appropriate strength (dosage) and route of administration.
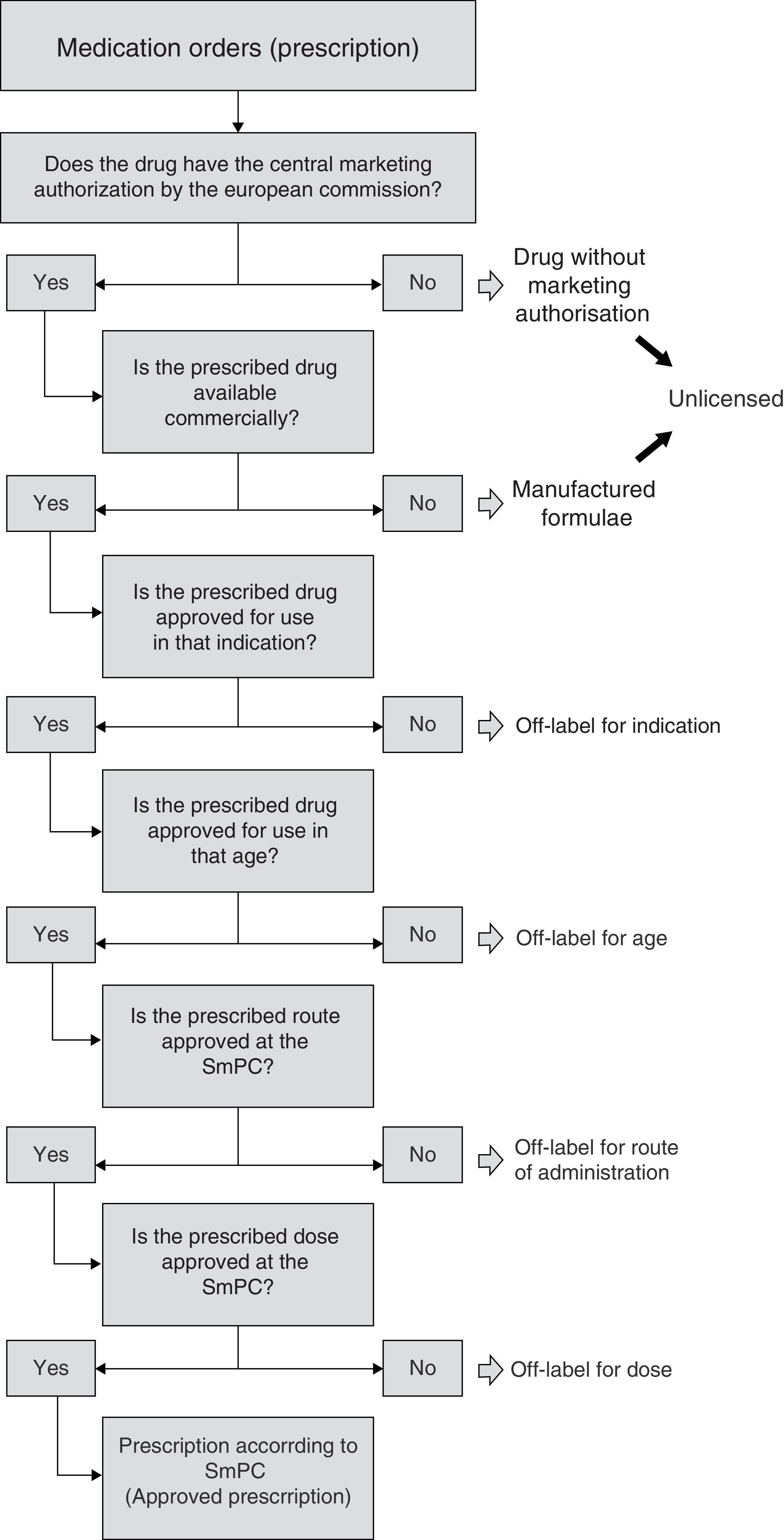
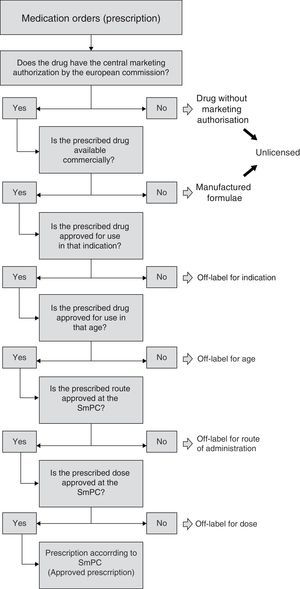
In this way, and for uniform consideration, having revised all the relevant bibliography, an algorithm was defined allowing sequential observation of information included in every prescription, with the aim of defining prescription condition (Fig. 1). The first step was to check that the included drug had European Marketing Authorisation. Drugs without Marketing Authorisation were to be considered “unlicensed” (i.e. investigational drugs). In addition, we also judged as “unlicensed” the drugs used as special formulations, i.e. the formulations manufactured by the Hospital Pharmacy Service (“manufactured formulae”)16, considering that the manipulation of the drug as well as the lack of information on the acquired physicochemical properties could affect relevant pharmacological characteristics. On the other hand, when dealing with a commercialized drug, the condition according to indication was evaluated, whether it was authorized or not for the age group, route of administration and finally according to dose. That is to say, commercialized drugs, in this study, were classified under one possible reason for off-label use. As regards hospital pharmacy “manufactured formulae”, mainly classified as unlicensed, their possible off-label use according to indication, age or other reasons was evaluated in a secondary manner according to the algorithm.
The statistical analysis of data was performed with the SPSS 15.0 (IBM Corporation, Armonk, NY, USA) package. The Chi-square lineal tendency test was used for lineal association between variables.
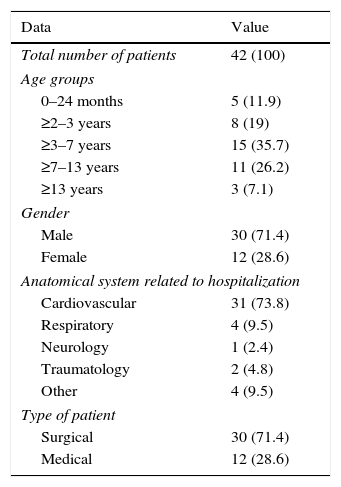
ResultsDuring a six-week study, data from a total of 42 patients were collected. Patient characteristics included in the study are shown in Table 1. Of these, 696 prescriptions were analyzed, involving a total of 102 different drugs, mainly belonging to the group for nervous system therapy (N: 36.1%), cardiovascular system (21.6%) and digestive system (A: 10.5%). The most prescribed drugs were fentanyl (7.9%), midazolam (7.9%), atropine (5.2%), etomidate (5.2%) and suxamethonium (5.2%).
Patient characteristics.
| Data | Value |
|---|---|
| Total number of patients | 42 (100) |
| Age groups | |
| 0–24 months | 5 (11.9) |
| ≥2–3 years | 8 (19) |
| ≥3–7 years | 15 (35.7) |
| ≥7–13 years | 11 (26.2) |
| ≥13 years | 3 (7.1) |
| Gender | |
| Male | 30 (71.4) |
| Female | 12 (28.6) |
| Anatomical system related to hospitalization | |
| Cardiovascular | 31 (73.8) |
| Respiratory | 4 (9.5) |
| Neurology | 1 (2.4) |
| Traumatology | 2 (4.8) |
| Other | 4 (9.5) |
| Type of patient | |
| Surgical | 30 (71.4) |
| Medical | 12 (28.6) |
Figures in parentheses indicate percentage.
As regards treatments, patients received an average of 16.5 prescriptions (median: 14, SD: 9.3; P25: 11; P75: 21). The most “simple” patient received only four different drug prescriptions, whereas the most “complex” patient received 48 different prescriptions throughout hospitalization.
Of all different drugs involved (102), a total of 54 (52.9%) drugs were identified as being prescribed off-label and 16 (15.7%) as unlicensed.
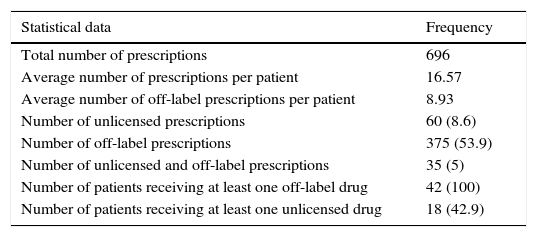
All patients received at least one off-label prescription and 18 patients at least one unlicensed one. Only one patient received only one off-label drug, whereas the others (41) received more than one. The mean of drugs used under off-label conditions per treatment and per patient was 8.9 (median: 7; SD: 5.8; P25: 5; P75: 12). The summary of statistical parameters relative to prescription analysis is shown in Table 2.
Summary of parameters analyzed.
| Statistical data | Frequency |
|---|---|
| Total number of prescriptions | 696 |
| Average number of prescriptions per patient | 16.57 |
| Average number of off-label prescriptions per patient | 8.93 |
| Number of unlicensed prescriptions | 60 (8.6) |
| Number of off-label prescriptions | 375 (53.9) |
| Number of unlicensed and off-label prescriptions | 35 (5) |
| Number of patients receiving at least one off-label drug | 42 (100) |
| Number of patients receiving at least one unlicensed drug | 18 (42.9) |
Figures in parentheses indicate percentage.
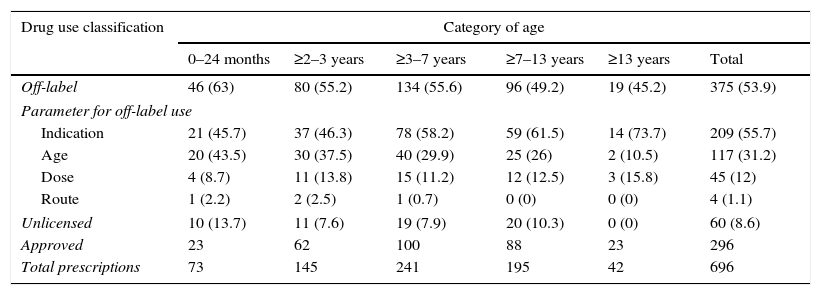
In agreement with the established algorithm, 340 prescriptions were initially used as off-label for different reasons, and 60 prescriptions were used under unlicensed conditions (8.6% of the total prescriptions, all being pharmacy-manufactured formulae), of which 35 (5% of all prescriptions) were considered off-label as well. Therefore, the global results show an off-label use of 375 prescriptions (53.9%): 55.7% (209 prescriptions) due to indication different to the approved one, 31.2% (117 prescriptions) age different to the age authorized, 12.0% (45 prescriptions) “non-approved” dose and finally 1.1% (4 prescriptions) route of administration not contemplated on SmPC. The use of drugs under approved conditions was seen in 296 prescriptions (42.5%).
The sub-analysis by age group showed that for children under two years, 63% of their prescriptions, corresponded to off-label use, and 13.7% to unlicensed use; in higher age groups, the off-label and unlicensed percentages tend to decrease, dropping to 45.2% and 0% respectively in the case of adolescents (≥13 years). The Chi-square lineal tendency test was significant (p=0.025), thus verifying the existing lineal association between frequency of off-label use and patient age; however, this lineal tendency was not observed in the case of unlicensed drug use, possibly due to lower sample size.
The reasons for using drugs under off-label conditions were independent of patient age; we found the same results as in the global analysis: indication was the first reason for off-label use in all groups, followed by age, dose and finally route of administration (Table 3).
Distribution of off-label, unlicensed and approved drug use by patient age group.
| Drug use classification | Category of age | |||||
|---|---|---|---|---|---|---|
| 0–24 months | ≥2–3 years | ≥3–7 years | ≥7–13 years | ≥13 years | Total | |
| Off-label | 46 (63) | 80 (55.2) | 134 (55.6) | 96 (49.2) | 19 (45.2) | 375 (53.9) |
| Parameter for off-label use | ||||||
| Indication | 21 (45.7) | 37 (46.3) | 78 (58.2) | 59 (61.5) | 14 (73.7) | 209 (55.7) |
| Age | 20 (43.5) | 30 (37.5) | 40 (29.9) | 25 (26) | 2 (10.5) | 117 (31.2) |
| Dose | 4 (8.7) | 11 (13.8) | 15 (11.2) | 12 (12.5) | 3 (15.8) | 45 (12) |
| Route | 1 (2.2) | 2 (2.5) | 1 (0.7) | 0 (0) | 0 (0) | 4 (1.1) |
| Unlicensed | 10 (13.7) | 11 (7.6) | 19 (7.9) | 20 (10.3) | 0 (0) | 60 (8.6) |
| Approved | 23 | 62 | 100 | 88 | 23 | 296 |
| Total prescriptions | 73 | 145 | 241 | 195 | 42 | 696 |
Note: The sum of off-label and unlicensed, in each age group, does not coincide with the total prescriptions, as one must take into account those 35 unlicensed prescriptions which also fulfil off-label requisites. () percentage based on the total items prescribed for each age group; percentage of different categories of off-label use based on the total off-label prescriptions for each age group.
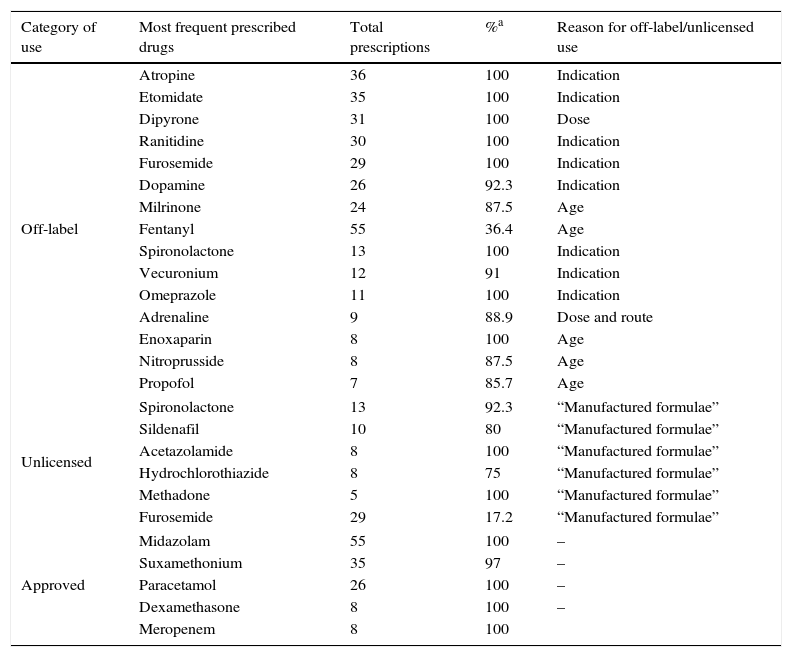
Regarding the drugs involved in prescriptions, Table 4 shows the drugs mainly used in this PICU in their different use categories, indicating the total number of prescriptions for each and percentage of off-label, unlicensed or approved prescriptions respectively. When analysing results according to reason for off-label use, the drugs most frequently used with different indication to that approved were: atropine, etomidate, ranitidine, furosemide and dopamine; off-label due to patient age: milrinone, fentanyl, enoxaparin and clorazepate; due to dose: dipyrone and adrenaline, and due to route of administration: adrenaline, vancomycin and morphine. In the unlicensed drug setting, spironolactone (20%), sildenafil (13.3%), acetazolamide (13.3%) and hydrochlorothiazide (10%) stand out in 100% of the cases, which were “manufactured formulae”.
Most common drugs in different category of use in PICU.
| Category of use | Most frequent prescribed drugs | Total prescriptions | %a | Reason for off-label/unlicensed use |
|---|---|---|---|---|
| Off-label | Atropine | 36 | 100 | Indication |
| Etomidate | 35 | 100 | Indication | |
| Dipyrone | 31 | 100 | Dose | |
| Ranitidine | 30 | 100 | Indication | |
| Furosemide | 29 | 100 | Indication | |
| Dopamine | 26 | 92.3 | Indication | |
| Milrinone | 24 | 87.5 | Age | |
| Fentanyl | 55 | 36.4 | Age | |
| Spironolactone | 13 | 100 | Indication | |
| Vecuronium | 12 | 91 | Indication | |
| Omeprazole | 11 | 100 | Indication | |
| Adrenaline | 9 | 88.9 | Dose and route | |
| Enoxaparin | 8 | 100 | Age | |
| Nitroprusside | 8 | 87.5 | Age | |
| Propofol | 7 | 85.7 | Age | |
| Unlicensed | Spironolactone | 13 | 92.3 | “Manufactured formulae” |
| Sildenafil | 10 | 80 | “Manufactured formulae” | |
| Acetazolamide | 8 | 100 | “Manufactured formulae” | |
| Hydrochlorothiazide | 8 | 75 | “Manufactured formulae” | |
| Methadone | 5 | 100 | “Manufactured formulae” | |
| Furosemide | 29 | 17.2 | “Manufactured formulae” | |
| Approved | Midazolam | 55 | 100 | – |
| Suxamethonium | 35 | 97 | – | |
| Paracetamol | 26 | 100 | – | |
| Dexamethasone | 8 | 100 | – | |
| Meropenem | 8 | 100 | ||
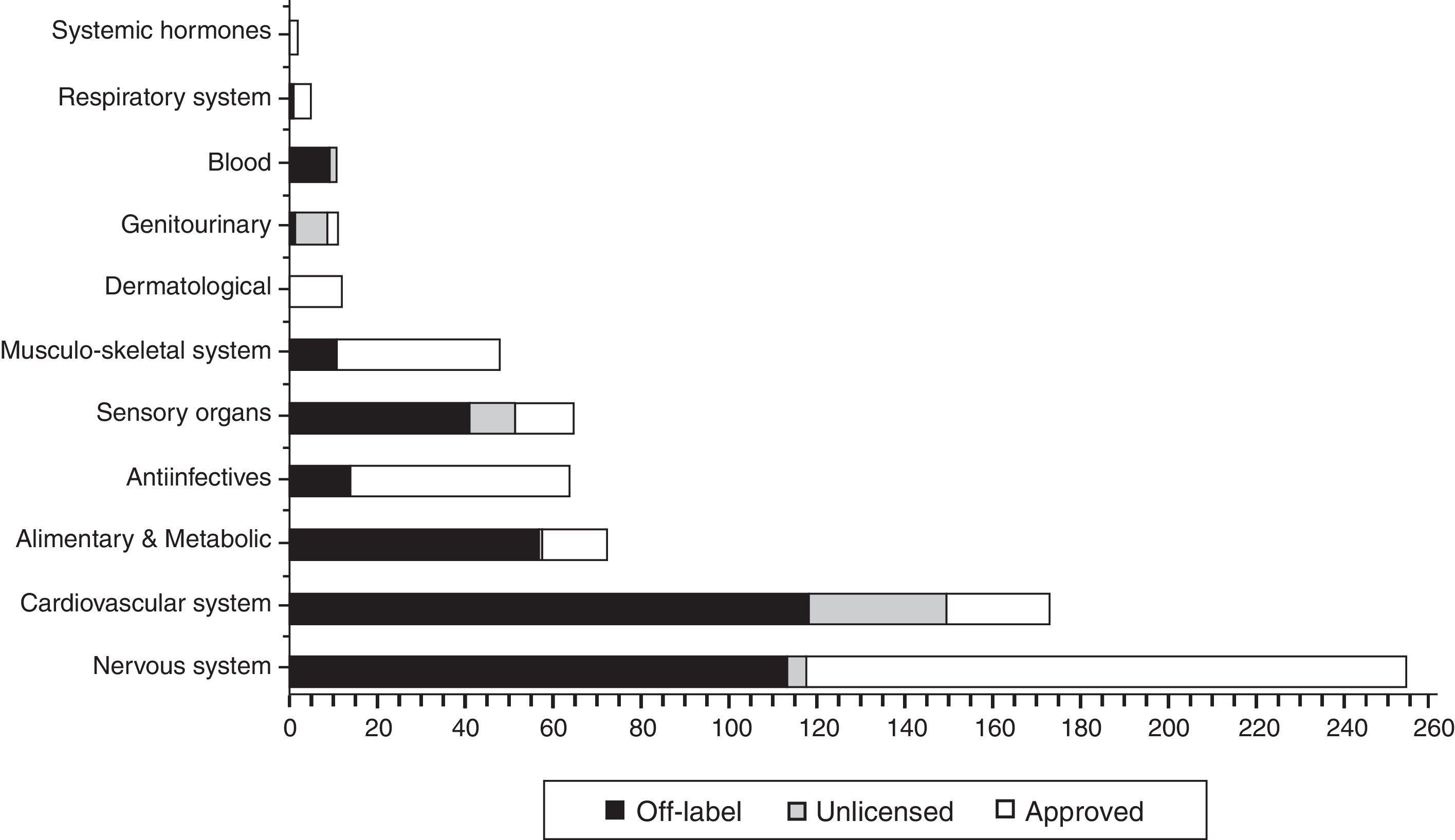
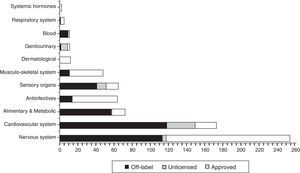
In Fig. 2 the distribution is shown the total, in each ATC group (World Health organisation Anatomical Therapeutic Chemical classification system), of drugs prescribed according to their use category, highlighting the fact that most drugs used under “non-approved” conditions (off label and unlicensed) are concentrated in group B: blood (100%), Group C: cardiovascular system (84.7%), Group G: genitourinary (83.3%), Group A: digestive (79.5%) and Group S: sensory organs (79.4%).
DiscussionThe present project is encompassed within a common European strategy, established according to Paediatric Regulation articles 42 and 43 – Regulation (EC) N° 1901/2006 of the European Parliament and Congress on paediatric drug use. This regulation was published with the intention of facilitating development and improving accessibility of drugs in paediatric populations as well as to ensure that drugs used in treating children undergo high quality research and are correctly authorized for use in this population.
One of the legal requirements of this Paediatric Regulation is to collect all available data on all uses of drugs in paediatric populations. Three non-European Union members were invited to participate in this process (Iceland, Norway and Liechtenstein). The report on the use profile, off-label as well as unlicensed in all Europe,17 highlights, as one of its limitations, that some countries representing approximately 50% of the European paediatric population have not presented their results and therefore the use profile is lacking that data.
On the other hand, studies performed in such specific and complex sub-populations as critical paediatric patients are few. Nevertheless, all show that paediatric intensive care units are characterized by a high ratio of drugs prescribed in “non-approved” conditions.18–28 We obtained an off-label use profile of 53.9% in prescriptions and 8.6% unlicensed use, similar to results in other studies.
When comparing with other PICU studies, it must be mentioned that although in our work the time and population may seem low, the final sample obtained (total number of prescriptions analyzed), is very similar to other studies.18–22
Turner et al. (Great Britain)18 having studied drug use conditions in a PICU, in 1996, published that 31% of all prescriptions involved drug use outside approved conditions (unlicensed or off-label) and that 70% of the total of patients received at least one drug under these conditions. Their percentages were slightly lower than those obtained in other posterior studies, but without doubt this is significant. A study with similar characteristics to ours, performed by Carvalho et al.,19 where over a six-week period a total of 747 drug prescriptions were analyzed (n=51), showed that 60% of those prescriptions involved drugs used in “non-approved” conditions, and that 88% of the total patients were prescribed at least one drug under these conditions. In Israel, Gavrilov et al.20 concluded that 81% corresponded to “non-approved use” and 83% of the patients received some drugs used in conditions different to those authorized. Ferreira et al.21 analyzed 1,054 prescriptions items in a Brazilian hospital (2011) and obtained 23.4% of the total prescribed items were off-label and 12.6% were unlicensed. The off-label percentage, in this survey, is lower than other similar studies in PICUs.
A current survey has been published by Blanco-Reina et al.22 in Spain (2015), which showed results similar to our study. They assessed the prescription profile and license status of drugs used in a neonatal and paediatric intensive care unit; they analyzed a total of 601 prescriptions, concluding that a little over one-half of the prescriptions (52% of the total) were off-label. This study is particularly relevant because it provides data of our geographic area. The difference is that Blanco-Reina includes neonatal patients.
Other published studies: In India (2006), Bavdekar et al.23 also determined off-label use in a PICU; a large study was performed, with similar results: 70.6% off-label drug use, and in Malaysia, Lee et al.24 designs a wide study to determine the extent of unlicensed and off-label use of drugs in children admitted to the 3 intensive care units for 8 weeks. Out of the 1,295 prescriptions, 34.1% were off-label and 27.3% were unlicensed.
In spite of the concordance of the results presented, there are also differences with other published studies, for example the study by 't Jong et al.,25 where the total of unlicensed prescriptions reached 52% and off-label 16%. These results are justified by the high percentage of medications manufactured by the Pharmacy Hospital Service (35%) and the criteria established in this study to define a drug as unlicensed, which without doubt are much broader than those established in similar studies.
In general, we can conclude that approximately 55% of all prescriptions (between 23% and 81%) and 80% of all patients (between 70% and 88%) receive drugs outside conditions approved in these units, data which mainly agree with the situation found in our study. Nevertheless, one aspect where significant differences were found was the reason for using drugs off-label. In other similar studies, dose was the principal reason for off-label use19,21–23,25 followed by age23 or frequency of administration19; in our study, the main reason was drug indication followed by age and lastly dose. These differences may be due to several reasons, such as different medical prescription practices depending on geographic area, which is obvious from the high heterogeneity existing amongst frequently used drugs in each country; bibliographic references used in each study to evaluate drug use conditions; or even the methodology of our own study, as every commercialized drug is assigned only one possible off-label use (according to the established algorithm), whereas in other studies19,22,23,25 all possible reasons for off-label use of a drug are taken into account and evaluated. Nevertheless, due to the low number of studies evaluating this variable and therefore lack of information on this issue, the data must be interpreted with caution.
The analysis of off-label or unlicensed use frequency in different age groups is poorly documented and results obtained to date are not conclusive. Carvalho et al.19 did not find significant differences amongst the different age groups. Bavdekar23 and Ferreira,21 in agreement with our results, place the highest off-label use percentages within the lowest age groups.
Regarding the drugs most used in “non-approved” conditions in a PICU, currently published data show great variability, motivated, perhaps, by differences in medical practice within different geographical areas, different disease profiles attended in each PICU and the progressive advances in medication use in paediatrics. Nevertheless, dopamine, furosemide, ranitidine or adrenaline, amongst others, are some of the off-label drugs that are most often found published in PICUs, also included in our results.
This is a pilot study, conducted in a small population and designed to analyze some methodological aspects, prior to the definitive study. Consequently, from this pilot study, we have been able to extract several possible areas for improvement in future researches, e.g.: add a new variable drug from which to collect frequency of administration; or consider the drugs that have to be imported from others countries as unlicensed, not as approved (drugs without marketing authorization in our environment, Spain, and that therefore should not be considered in the same way for marketing authorization as drugs from Spain).
On the other hand, this study has its limitations, similar to other studies, it does not cover all seasons and therefore there may be medications not covered here, especially those used in specific seasonal illnesses. However, the main limitation of our study is perhaps the obvious cardiac profile of the patients hospitalised in our paediatric intensive care unit. The different medical or surgical profiles or disease profiles of intensive care units may lead to different results in their studies, and PICUs like ours, that treat patients with cardiovascular surgery tend to use higher numbers of off-label and unlicensed drugs.26,27
Thus, this allows comparison with our most familiar environment and, in conjunction, permits us to find the areas for improvement, marked by this regulation, to guarantee effective, safe and efficient drug use in children. Studies like this may provide valuable information to drug agencies to prioritize researches of certain drugs in the paediatric population, particularly those most frequently used in an off-label or unlicensed manner.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García-López I, Fuentes-Ríos JE, Manrique-Rodríguez S, Fernández-Llamazares CM. Utilización de medicamentos en condiciones off-label y unlicensed: resultados de un estudio piloto realizado en una unidad de cuidados intensivos pediátricos. An Pediatr (Barc). 2017;86:28–36.