To determine whether the availability of heated humidified high-flow nasal cannula (HFNC) therapy was associated with a decrease in need for mechanical ventilation in neonates hospitalised with acute bronchiolitis.
MethodsA combined retrospective and prospective (ambispective) cohort study was performed in a type II-B Neonatal Unit, including hospitalised neonates with acute bronchiolitis after the introduction of HFNC (HFNC-period; October 2011–April 2015). They were compared with a historical cohort prior to the availability of this technique (pre-HFNC; January 2008–May 2011). The need for mechanical ventilation between the two study groups was analysed. Clinical parameters and technique-related complications were evaluated in neonates treated with HFNC.
ResultsA total of 112 neonates were included, 56 after the introduction of HFNC and 56 from the period before the introduction of HFNC. None of the patients in the HFNC-period required intubation, compared with 3.6% of the patients in the pre-HFNC group. The availability of HFNC resulted in a significant decrease in the need for non-invasive mechanical ventilation (30.4% vs 10.7%; P=.01), with a relative risk (RR) of .353 (95% CI: .150–.829), an absolute risk reduction (ARR) of 19.6% (95% CI: 5.13–34.2), yielding a NNT of 5. In the HFNC-period, 22 patients received high flow therapy, and 22.7% (95% CI: 7.8–45.4) required non-invasive ventilation. Treatment with HFNC was associated with a significant decrease in heart rate (P=.03), respiratory rate (P=.01), and an improvement in the Wood-Downes-Férres score (P=.00). No adverse effects were observed.
ConclusionsThe availability of HFNC reduces the need for non-invasive mechanical ventilation, allowing a safe and effective medical management of neonates with acute bronchiolitis.
Determinar si el uso de oxigenoterapia de alto flujo (OAF) en cánulas nasales disminuye la necesidad de ventilación mecánica en neonatos hospitalizados con bronquiolitis aguda.
MétodosEstudio de cohortes ambispectivo, realizado en una unidad neonatal IIB, que incluyó neonatos ingresados con bronquiolitis desde la instauración de la técnica de OAF (período-OAF: octubre de 2011-abril de 2015), comparándolo con una cohorte histórica de la temporada previa a su uso (período pre-OAF: enero de 2008-mayo de 2011). Se analizó la proporción de ventilación mecánica antes y después del inicio del tratamiento con OAF y se evaluaron parámetros clínicos y complicaciones de los pacientes tratados con esta técnica.
ResultadosSe incluyeron 112 neonatos, 56 del período-OAF y 56 de la temporada pre-OAF. En el período-OAF ningún paciente requirió intubación en comparación con la temporada previa, donde el 3,6% precisó ventilación mecánica invasiva. El uso de OAF se asoció con una disminución significativa de ventilación mecánica no invasiva (30,4% vs 10,7%; p=0,01), con un RR de 0,353 (IC 95%: 0,150-0,829), RAR de 19,6% (IC 95%: 5,13-34,2) y NNT de 5. En el período-OAF 22 pacientes recibieron terapia de alto flujo y 22,7% de ellos (IC 95%: 7,8-45,4) requirieron ventilación no invasiva. Tras el inicio de OAF se observó una mejoría rápida y progresiva de la frecuencia cardiaca (p=0,03), frecuencia respiratoria (p=0,01) y escala clínica (p=0,00) a partir de 3h. No se registraron efectos adversos.
ConclusionesEl uso de OAF disminuye la necesidad de ventilación no invasiva y es un tratamiento seguro que consigue mejoría clínica de neonatos con bronquiolitis.
Acute bronchiolitis is the respiratory disease that is the most frequent cause of hospitalisation during the winter months.1,2 Although most cases are self-limiting and can be managed in the home, between 1 and 5% require hospital admission, and of the latter, 5–15% require respiratory support at the paediatric intensive care unit (PICU).2–6 Age less than 6 weeks is a risk factor for severity, and approximately 30–50% of patients admitted to the PICU are less than 1 month of age.4
The treatment of bronchiolitis remains a controversial subject. There is no evidence of any treatment being capable of altering the natural course of the disease, but some treatments may prevent the development of complications and improve patient comfort. The approaches to the management of these patients backed by scientific evidence consist of supportive care and mechanical ventilation.2,6–10 In recent years, clinical practice has widely incorporated the use of nebulised hypertonic saline (HTS) in moderate bronchiolitis and of noninvasive ventilation (NIV) and high-flow nasal cannula (HFNC) oxygen therapy as supportive measures to prevent invasive mechanical ventilation (IMV) in patients with severe bronchiolitis.6,11–20 Although NIV has proven to be a useful tool in paediatric patients with respiratory failure, its use may be limited in low birth weight infants due to their poor tolerance of the technique.21 High-flow nasal cannula therapy is a noninvasive respiratory support technique that delivers a heated and humidified blend of air and oxygen through nasal cannulae at rates exceeding the peak inspiratory flow, and has proven useful in the management of moderate to severe bronchiolitis.14,15,22–27 The effectiveness of this technique compared to CPAP has been assessed in newborns for the treatment of neonatal respiratory distress syndrome,28–32 but there is little published evidence on its use in newborns with bronchiolitis.32
The aim of this study was to determine whether initiation of HFNC oxygen therapy with nasal cannulae in a neonatal unit succeeded in reducing the need for mechanical ventilation in newborns admitted with acute bronchiolitis. We also analysed the clinical outcomes of patients treated with HFOT, as well as the complications that developed.
Patients and methodsWe conducted an ambispective cohort study in the level IIB neonatal unit of the Consorcio Hospital General Universitario de Valencia, Spain, which included: (1) a prospective cohort of newborns admitted with bronchiolitis from October 2011, when the use of HFNC oxygen therapy was introduced in the unit, to April 2015 (HFNC period); (2) comparison with a retrospective cohort of newborns admitted with bronchiolitis in the period preceding the introduction of HFNC, from January 2008 to May 2011 (pre-HFOT period).
The diagnosis of bronchiolitis was made following the definition proposed by McConnochie: first episode of respiratory distress with wheezing, preceded by signs of viral respiratory illness.33 The inclusion criteria were chronological age of 28 days or less in term newborns or corrected gestational age of 42 weeks or less in preterm newborns (<37 weeks’ gestation). We excluded patients with haemodynamic instability, sepsis, or that required intubation on admission.
The primary outcome variable was the need for mechanical ventilation (NIV and IMV) in the two periods under study. We analysed other variables, such as sex, gestational age, birth weight, age and weight at the time of admission, aetiological agent, infant feeding method, prior history of palivizumab treatment, comorbidities, pharmacological treatment received during admission, length of stay and need for transfer to the PICU.
The study was approved by the Research Commission of the Consorcio Hospital General Universitario de Valencia.
High flow nasal cannula oxygen therapy periodHFNC oxygen therapy was introduced with a protocol based on the existing scientific evidence that set the criteria for its use as well as step-wise criteria to transition to other modalities of respiratory support when required (NIV or IMV). Continuous monitoring by pulse oximetry and supportive care were initiated in all patients, while the need for pharmacological treatment with nebulised adrenaline was assessed on a case-by-case basis, following the same protocol applied in preceding years. Respiratory support with HFNC oxygen therapy was initiated if patients met any of the criteria listed in Table 1.
Criteria for initiation of different respiratory support modalities.
| Criteria for use of high-flow nasal cannula oxygen therapy |
| Wood-Downes-Ferrés score>6 |
| Requires oxygen therapy with conventional nasal prongs at >2L/min to maintain an oxygen saturation>92% |
| Respiratory acidosis with hypercapnia>50mmHg in capillary blood gas |
| Apnoeas |
| Criteria for initiation of noninvasive mechanical ventilation |
| Persistent apnoeas |
| Hypercapnia with pH of 7.20–7.25 |
| Requires FiO2>0.40 to achieve SaO2>92% |
| Criteria for initiation of invasive mechanical ventilation |
| Symptoms of severe respiratory distress with signs of imminent respiratory failure |
| Persistent apnoeas despite NIV |
| Requires FiO2>0.6 to achieve SaO2>90% |
| Altered level of consciousness |
HFNC oxygen therapy was delivered using the Fisher & Paykel® MR850® system with nasal cannulae (Optiflow™). Therapy was initiated at a rate of 4–6L/min that was increased progressively to a maximum of 10L/min until clinical improvement was achieved. The initial fraction concentration of oxygen in inspired gas (FiO2) was set at the pressure required to achieve an oxygen (SaO2) of more than 92% and was adjusted based on how the patient responded to a maximum FiO2 of 0.40. If the patient became clinically stable with a Wood-Downes-Ferrés (WDF) score of 4 or higher, the flow rate was gradually reduced to 2L/min and the FiO2 to 0.25, and HFNC oxygen therapy was discontinued. At this point, the need for oxygen therapy with conventional nasal prongs at 2L/min or less was assessed.
Failure of HFNC oxygen therapy was defined as lack of clinical improvement or worsening in the condition of the patient despite the optimisation of therapy with delivery of maximum FiO2 and flow rates, requiring transition to another modality of respiratory support. Table 1 presents the criteria for initiation of NIV or IMV. The need for IMV was considered a criterion for admission to the PICU or the neonatal ICU.
During treatment with HFNC oxygen therapy, we documented the following parameters at different time points: temperature, respiratory rate, heart rate (HR), SaO2, FiO2 and WDF score. The parameters were recorded at the time of initiation of HFNC oxygen therapy (baseline) and at 3, 6, 12, 18, 24, 36, 48, 72 and 96h. We recorded capillary blood gas parameters (pH, PCO2) at 6, 12, 18, 24, and 48h from initiation of HFNC oxygen therapy, and documented adverse effects (pneumothorax, intolerance to the technique, hypothermia, skin erosion at the area of contact with the cannula, or aspiration of food), duration of nothing by mouth (NPO), and method of feeding.
Pre-high-flow oxygen therapy periodIn the second phase of the study, we collected data retrospectively by reviewing the medical records of newborns admitted with bronchiolitis over a period of four epidemic seasons preceding the introduction of HFNC oxygen therapy in the unit, applying the inclusion and exclusion criteria. During this period, the criteria for hospital admission and the management of patients were similar to those applied in the HFNC period, with the exception of the use of HFNC oxygen therapy.
Statistical analysisWe performed the statistical analysis with the software package IBM SPSS 20.0 for Windows®. We have summarised categorical variables as percentages and 95% confidence intervals (CIs) and quantitative variables as mean±standard deviation in case they followed a normal distribution, or median and interquartile range otherwise.
We compared the baseline characteristics of the two cohorts by means of the Mann–Whitney U test for continuous variables and Fisher's exact test or the chi-square test for categorical variables. We assessed the impact of the introduction of HFNC oxygen therapy on mechanical ventilation by means of the following measures: relative risk (RR), absolute risk reduction (ARR) and the number needed to treat (NNT) with HFNC oxygen therapy to prevent the use of mechanical ventilation. When we analysed the length of stay, we excluded patients that were transferred to the PICU.
We analysed the changes in quantitative secondary outcome variables (WDF score, HR, respiratory rate, SaO2, pH, PCO2, flow rate in L/min) by means of the Mann–Whitney U test for paired samples. We defined statistical significance as a P-value of less than .05.
ResultsWe included a total of 112 newborns, 56 in the cohort of the HFNC period (2011–2015) and 56 in the cohort of the period preceding the introduction of HFNC oxygen therapy (2008–2011). We did not find statistically significant differences between the cohorts in the baseline characteristics of the patients or the pharmacological treatment they received (Table 2).
Characteristics of the newborns in the two periods under study: pre-HFNC (2008–2011) and HFNC (2011–2015).
| Pre-HFNC group (n=56) | HFNC group (n=56) | P | ||
|---|---|---|---|---|
| Age (days) | Median | 20 [15.2–26.9] | 21.6 [17.1–26.3] | .59 |
| Birth weight (g) | Median | 3200 [2900–3650] | 3265 [2965–3627.5] | .76 |
| Weight at admission (g) | Median | 3490 [3013.8–3803.8] | 3597.5 [3276.3–3993.8] | .12 |
| Gestational age (weeks) | Median | 39.3 [1.38–40] | 39.2 [3.38–40] | .78 |
| Preterm birth (%) | 17.9 (6.928.8) | 16.1 (5.626.6) | .80 | |
| Sex (male) (%) | 53.6 (39.6–67.5) | 50.9 (36–64) | .78 | |
| Respiratory syncytial virus (%) | 78.6 (66.9–90.2) | 78.6 (66.9–90.2) | 1 | |
| Palivizumab (%) | 5.4 (1.1–14.9) | 5.4 (1.1–14.9) | 1 | |
| Breastfeeding (%) | 50 (36–64) | 60.7 (47–74.4) | .25 | |
| Bronchopulmonary dysplasia | 0 | 0 | ||
| Acyanotic heart disease (%) | 1.8 (0.1–9.5) | 5.4 (1.1–14.9) | .62 | |
| Neuropathy (%) | 1.8 (0.1–9.5) | 0 | 1 | |
| Antibiotic treatment (%) | 32.1 (19.2–45.3) | 26.8 (14.3–39.3) | .53 | |
| Nebulised adrenaline (%) | 67.9 (54.7–81) | 78.6 (66.9–90.2) | .20 | |
| Nebulised salbutamol (%) | 16.1 (5.6–26.6) | 12.5 (3–22.1) | .59 | |
| Nebulised 3% hypertonic saline (%) | 17.9 (6.9–28.8) | 14.3 (4.2–24.3) | .61 | |
| Respiratory support (%) | ||||
| No | 19.6 (8.3–30.9) | 33.9 (20.6–47.2) | .09 | |
| O2 nasal cannulae | 50 (36–64) | 25 (12.8–37.2) | .01 | |
| HFNC | 0 | 39.3 (25.6–53) | .00 | |
| NIV | 30.4 (17.4–43.3) | 10.7 (1.7–19.7) | .01 | |
| IMV | 3.6 (0.4–12.3) | 0 | .50 | |
| Length of stay in days | Median | 5.92 [4.46–7.51] | 6.03 [4.68–7.94] | .69 |
Categorical variables are expressed as percentage (95% CI) and quantitative variables as median [interquartile range].
The respiratory support techniques used in each period were different, and we observed that the use of HFNC oxygen therapy was associated with a significant reduction in the need for NIV (30.4% vs 10.7%; P=.01), RR of 0.35 (95% CI: .15–.83), ARR of 19.6% (95% CI: 5.13–34.20) and NNT of 5. We did not find a significant difference in the use of IMV. The length of stay in days was similar in both groups (5.92 pre-HFOT vs 6.03 in the HFOT period; P=.688) (Table 2).
We analysed the data for the patients that received respiratory support with HFNC oxygen therapy in the 2011–2015 period (n=22). Table 3 shows their baseline characteristics and the treatments they received.
Characteristics of patients that received respiratory support with HFOT in the 2011–2015 period.
| N=22 | 95% CI [IQR] | |
|---|---|---|
| Age (days) [median] | 20.8 | [16.9–25.6] |
| Gestational age (weeks) [median] | 39.2 | [38.1–40.3] |
| Preterm birth (%) | 4 (18.2) | 5.2–40.3 |
| Birth weight (g) [median] | 3.235 | [2.800–3.500] |
| Weight at admission (g) [median] | 3.525 | [3.280–3.870] |
| Sex (male) (%) | 13 (59.1) | 36.3–81.9 |
| Respiratory syncytial virus (%) | 21 (95.5) | 77.2–99.9 |
| Family history of atopy (%) | 5 (22.7) | 7.8–45.4 |
| Previous admission (%) | 9 (40.9) | 18.1–63.7 |
| Acyanotic heart disease (%) | 1 (4.5) | 0.1–22.8 |
| Breastfeeding (%) | 11 (50) | 26.8–73.2 |
| Palivizumab (%) | – | – |
| Fever (%) | 14 (63.6) | 41.3–86 |
| Atelectasis/consolidation (%) | 11 (50) | 26.8–73.2 |
| Antibioterapia (%) | 11 (50) | 26.8–73.2 |
| Nebulised adrenaline (%) | 22 (100) | 84.5–100 |
| Nebulised salbutamol (%) | 1 (4.5) | 0.1–22.8 |
| Nebulised 3% hypertonic saline (%) | 4 (18.2) | 5.2–40.3 |
| Clinical parameters prior to initiation of HFNC [median] | ||
| Clinical score | 7 | [7–8] |
| Heart rate | 160 | [150–170] |
| Respiratory rate | 60 | [48–70] |
| Oxygen saturation | 94 | [91–97] |
| FiO2 | 24 | [21–27] |
| pH (capillary blood gas) | 7.34 | [7.32–7.38] |
| PCO2 (capillary blood gas) | 57 | [52–60.5] |
Values expressed as percentage (95% CI) and median (interquartile range [IQR]).
High-flow nasal cannula oxygen therapy was initiated with a mean flow rate of 6.8L/min (SD, 1.5). The flow rate was increased gradually until reaching the maximum value (mean, 8.1L/min; SD, 1.7) at 12h, as can be seen in Fig. 1. The maximum flow administered was 10L/min at a dose of 3L/kg/min, with a maximum FiO2 of 0.40.
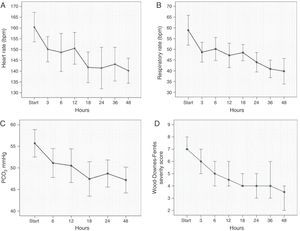
Treatment with HFNC oxygen therapy was associated with significant decreases in HR from 160±15.4 to 150.2±11.6 (P=.03), in respiratory rate from 59±14.6 to 48.7±8.6 (P=.01) and in the WDF score from 7 (6.8–8.3) to 6 (5–7) (P=.001), starting at 3h from initiation of HFNC oxygen therapy. We also observed a significant decrease in capillary blood PCO2 from 55.7±7.2 to 51.1±6.8 (P=.012) at 6h. We observed a decreasing trend in the different parameters under study throughout the treatment with HFNC oxygen therapy with a peak in clinical stabilisation at 18 to 24h (HR, 141±14; respiratory rate, 44±8; PCO2, 48.7±6; WDF severity score, 4 [4–5]) (Fig. 2).
Changes in cardiorespiratory parameters and in the Wood-Downes-Ferrés severity score in the first 48h of treatment with HFNC oxygen therapy. (A) Heart rate (mean, 95% CI). (B) Respiratory rate (mean, 95% CI). (C) PCO2 obtained from capillary blood gas testing (mean, 95% CI). (D) Wood-Downes-Ferrés severity score (median, interquartile range).
We did not observe adverse effects such as pneumothorax, intolerance of HFNC oxygen therapy, hypothermia, skin erosion at the area of contact with the cannula or aspiration of food.
Enteral feeding by nipple feeding or orogastric tube was used in 86.3% of patients during treatment following a period of initial stabilisation with a median duration of NPO of 12.3h (7.8–25).
High-flow oxygen therapy failed in five patients (22.7%; 95% CI: 7.8–45.4), who required sequential treatment with NIV. The causes of failure were respiratory acidosis (60%), requirement of a FiO2 greater than 0.40 (20%) and increased breathing effort (20%). None of the patients required intubation or transfer to the PICU or neonatal ICU. During this period, one patient was treated with NIV without prior HFNC oxygen therapy due to meeting the severity criteria for NIV at the time of admission.
The median duration of HFNC oxygen therapy was 57h (34.5–80.1) and the median length of stay was 8.1 days (6.6–9.7).
DiscussionThe main finding of our study was a significant decrease in the use of NIV after the introduction of HFNC oxygen therapy in our neonatal unit. Furthermore, two patients required IMV during the pre-HFNC period, while none required intubation or transfer to the ICU after the introduction of HFNC oxygen therapy.
Several studies have demonstrated that NIV is a useful tool in the management of severe bronchiolitis, its main advantage being that it reduces the incidence of complications associated with IMV.12,17 However, its use may pose challenges in infants due to limitations related to the interface or to poor tolerance, with some of the patients requiring sedation.21,34 Furthermore, it requires a ventilator and in many instances, transfer to the ICU, which results in increased costs of treatment. For these reasons, we believe that a reduction in the use of NIV offers significant advantages in the management of patients with bronchiolitis in addition to a reduction of health care costs, although we did not analyse these variables in our study.
When it came to the characteristics of our sample, the newborns included in the study did not have relevant perinatal risk factors, such as bronchopulmonary dysplasia, severe prematurity or cyanotic heart disease, which could be accounted for by the level of care of the unit. Our sample was not representative of the neonatal population with risk factors for severe disease, so our findings should be interpreted with caution and may only be applicable to populations with similar characteristics.
Previous studies have demonstrated that HFNC oxygen therapy reduces the need for intubation from 23% to 9% in patients with bronchiolitis admitted to the PICU.21 A retrospective study that included children aged less than 24 months admitted to the PICU with different respiratory diseases found that in the subset of patients with bronchiolitis, 25% required NIV after HFNC oxygen therapy and 4% required IMV.23 Another prospective study assessed the use of HFNC therapy in patients with bronchiolitis in a paediatric ward and found a significant reduction in the percentage of admissions to the PICU compared to previous periods; 20% of the patients in the study required admission to the PICU following HFNC oxygen therapy (16% NIV and 4% IMV).25
Consistent with the existing literature, 22.7% of the patients in our study required NIV following HFNC oxygen therapy. However, the population and setting of our study are different. Some studies that have analysed the use of HFNC oxygen therapy in infants with bronchiolitis included newborns in their samples, but did not report specific findings for this group of patients.14,35 The vulnerability of newborns, which results from the immaturity of their immune system and entails a higher risk of severe bronchiolitis, makes this population markedly different from other populations studied in the past. Our study is one of the first to assess the use of HFNC oxygen therapy in the treatment of bronchiolitis exclusively in newborns, and while our data did not suffice to evince a reduction in the rate of intubation, they demonstrated that HFNC oxygen therapy is effective in newborns with bronchiolitis, as its use was associated with a reduced need for NIV (from 30.4% to 10.7%).
Studies conducted in PICUs have shown a reduction in length of stay with the use of HFNC therapy.22 Our study did not find a significant difference in length of stay between the two periods. One possible explanation is that we analysed length of stay excluding patients that needed transferring, as they were not followed up in our institution by our research team. This probably led to an underestimation of the length of stay in the pre-HFNC period, as several previous studies have found a positive correlation between length of stay and the need for intubation.22
The prospective phase of the study allowed us to analyse the outcomes of newborns treated with HFNC oxygen therapy. We observed a quick and gradual improvement in the HR, respiratory rate and severity score during treatment, with a period of increased clinical stability at 18–24h from its initiation. This improvement made it possible to give enteral feeds to 86.3% of the newborns after an initial stabilisation period.
On the other hand, there was a significant reduction in the PCO2 starting at 6h of treatment, a variable for which there is little data in the use of this technique. Previous studies have demonstrated that HFNC oxygen therapy achieves a PEEP of 4±1.99cmH2O, increasing the mean airway pressure.36,37 The improvement in clinical condition resulting from a reduction of respiratory muscle fatigue and lung volume recruitment could contribute to improving gas exchange, with the corresponding reduction in the PCO2.
In this cohort, HFNC oxygen therapy was initiated on patients with a median WDF score of 7, which is indicative of moderate severity. Early initiation of HFNC could be the key to achieve optimal results, as easing respiratory effort prevents progression to respiratory muscle fatigue and the development of atelectases.38
Although one study reported the cases of one infant and two children who experienced adverse effects from HFNC therapy,39 the patients in our study did not experience any complications at the delivered flow rates. Previous studies have shown that the administration of flows equal or greater than 2L/kg/min produces a mean laryngeal pressure of 4 or more cmH2O that is correlated with clinically relevant results, such as an improved breathing pattern.14 The flow administered in our cohort was the amount needed to achieve clinical improvement without the development of side effects.
The findings of this study must be interpreted taking into account its strengths and weaknesses. One of its limitations lies in its nonrandomised design, which could be a source of bias. In order to minimise the possibility of selection bias, we included all patients with bronchiolitis and developed a protocol that established the criteria for the use of HFNC oxygen therapy with the purpose of avoiding overtreatment with this technique. The supportive care measures and the indications for NIV used during the HFCN period were the same as those used in the preceding period, as we attempted to minimise the time-period bias. The analysis of the data showed that the cohorts were similar in their baseline characteristics and other treatments received, which allowed us to obtain results of acceptable validity. While we are aware that the design of the study implies a poor control of potential biases, our findings could serve as the starting point for future studies.
The main strength of the study is that it is one of the first in the literature that assesses the impact of the use of HFNC oxygen therapy in newborns with bronchiolitis. Considering that the neonatal period is clearly different from all other paediatric age groups, and that newborns are a high-risk group with a high rate of admissions to intensive care units during epidemic seasons, providing evidence on this group of patients is highly relevant.
We concluded that HFNC oxygen therapy oxygen delivery is an efficacious, easy to use, well-tolerated therapy that succeeds in reducing the frequency of NIV in newborns with bronchiolitis, in addition to facilitating enteral nutrition. Its easy implementation makes it an ideal technique for level I and II neonatal units, which could contribute to improving the management of resources by trying to reserve neonatal and paediatric intensive care units for patients that require intensive respiratory support.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Bermúdez Barrezueta L, García Carbonell N, López Montes J, Gómez Zafra R, Marín Reina P, Herrmannova J, et al. Oxigenoterapia de alto flujo con cánula nasal en el tratamiento de la bronquiolitis aguda en neonatos. An Pediatr (Barc). 2017;86:37–44.