The use of nonpharmacological measures to reduce pain during vaccination has been studied extensively in infants, but there are fewer studies on its effectiveness in older children and on the parental perception of pain in children.
MethodsWe conducted a multicentre, quasi-experimental interventional study with a control group. Patients: infants aged 2–11 months and children aged 4 years that attended routine vaccination appointments. Setting: Primary care. Intervention during vaccination: infants were breastfed and 4-year-old children blew a party horn. Control: vaccination performed following routine practice. Measurement: NIPS (Neonatal Infant Pain Scale) and duration of crying in infants, Wong-Baker FACES pain rating scale in older children and parents.
ResultsThe study included 125 children (intervention: 60; control: 65). There was a significant decrease in perceived pain in the intervention groups: NIPS score in infants, 3.8 ± 1.1 compared to 5.2 ± 0.7 (P < .001); Wong-Baker FACES score at 4 years of 3.3 ± 1.7 compared to 4.2 ± 1.6 (P = .042). These same differences in support of the intervention were reflected in the parental assessments (3.4 ± 1.3 vs 4.5 ± 1.5; P < .001). The correlation between child and parent scores was strongly positive: 0.7 (95% CI, 0.59−0.78). However, the duration of crying was longer in the intervention group.
ConclusionThe use of distraction techniques reduces pain in children and the pain perceived by parents in their children, thus increasing their satisfaction with the procedure.
La utilización de medidas no farmacológicas para disminuir el dolor en la vacunación se ha estudiado extensamente en lactantes, pero hay menos estudios sobre su efectividad en niños mayores y sobre la valoración de los padres del dolor observado en los niños.
MetodologíaEstudio multicéntrico, de intervención, cuasiexperimental con grupo control. Pacientes: Lactantes de 2 y 11 meses, y niños de 4 años que acuden para vacunación sistemática. Ámbito: Atención primaria. Intervención durante la inmunización: Los lactantes recibieron lactancia materna. Los niños de 4 años soplaron un matasuegras. El grupo control siguió la práctica habitual. Medición: escala NIPS (Escala de dolor infantil y neonatal) y duración del llanto en los lactantes. Dibujos faciales de Wong-Baker para los niños mayores y padres.
ResultadosParticiparon 125 niños (60 intervención; 65 control). Hubo una disminución significativade la sensación de dolor en los grupos de intervención: puntuación NIPS en lactantes (3,8 ± 1,1 frente a 5,2 ± 0,7 [P <,001]). Escala de Wong-Baker a los 4 años (3,3 ± 1,7 frente a 4,2 ± 1,6 [P =,042]). Esas mismas diferencias a favor de la intervención se observan en la valoración de los padres (3,4 ± 1,3 frente a 4,5 ± 1,5 [P <,001]. La correlación de las puntuaciones de niños y padresfuealtamente positiva: 0,7 (IC95%: 0,59–0,78). Sin embargo, el tiempo de duración del llanto fue mayor en el grupo intervención.
ConclusionesLa utilización de medidas distractoras consigue la disminución del dolor en los niños y la percepción del dolor de los padres disminuye, lo que aumenta la satisfacción de estoscon el procedimiento.
Nonpharmacological analgesia refers to the use of prophylactic and complementary interventions aimed at alleviating pain without the use of medication. The mechanisms of action of these interventions are heterogeneous. Some interventions induce the release of endogenous endorphins, others activate certain neuropeptide systems that have the final effect of enhancing the action of endogenous opiates, and still others are aimed at distracting from pain.1
In the healthy paediatric population, vaccination is the most frequently used painful procedure. Although there are multiple publications on techniques to prevent and manage the pain associated with vaccination, few professionals have actually integrated these methods in everyday clinical practice.2
To date, the most frequently used nonpharmacological methods to alleviate pain during vaccination have been breastfeeding and distraction measures.
As regards breastfeeding, the holding of the baby in the mother’s arms, providing warmth, the familiar maternal smell, protection and a sweet food (the baby’s usual nourishment) is associated with a reduction in external signs of pain. The potential indications for this approach are the customary heel prick tests and vaccinations in the first months of life, as well as any other discomforting procedure as long as the procedure allows breastfeeding the infant at the time when the pain is produced.3 Breastfeeding has been used for painful procedures, such as venepuncture, with satisfactory results.4
A review by the Advisory Committee on Vaccines of the Asociación Española de Pediatría (Spanish Association of Pediatrics) on the reduction of pain and stress during vaccination found that the methods that proved efficacious in reducing pain were breastfeeding or administration of sweet-tasting solutions in babies up to 18 months and distraction manoeuvres in children aged 2–12 years.5
To assess the feasibility and efficacy of these measures of analgesia or distraction in the process of administration of childhood vaccines at the primary care level, we conducted a prospective study with a control group. A novel contribution of the study is that in addition to the assessment of the paediatric care team (in children under 2 years) or the children themselves (in children older than 2 years), we also analysed the assessment made by the parents or guardians in both age groups.
Material and methodsWe conducted a quasi-experimental intervention study with a control group.
Study universe: children aged less than 5 years visiting the primary care centre to receive vaccines included in the official routine childhood immunization schedule.
Inclusion criteria: patients aged 2 months, 11 months and 4 years that visited participating primary care centres in the context of the Healthy Child Programme. We excluded children who were not accompanied by a parent/legal guardian, with chronic illness or for whom we did not obtain informed consent. In children aged less than 2 years, there was an additional requirement that they be accompanied by the breastfeeding mother.
Vaccines administered, as established in the Routine Childhood Immunization Schedule of the Autonomous Community of the Basque Country of 20196.
- •
At ages 2 months and 11 months, administration of the hexavalent vaccine (hepatitis B, diphtheria, tetanus, pertussis, poliovirus and Haemophilus influenzae) followed by the pneumococcal conjugate vaccine.
- •
At age 4 years, the measles-mumps-rubella (MMR) vaccine and the varicella vaccine.
Setting: paediatric primary care.
Intervention: different in children aged less and more than 2 years.
- 1
Age < 2 years (2 months and 11 months). Breastfeeding before, during and after administration of the vaccine.
- 2
Age > 2 years (4 years). Distraction measures. Children were instructed to blow on a party horn when the vaccine was being administered.
Children were allocated to the intervention or control groups based on their primary care centre. In the case of infants, the intervention group included those centres in which breastfeeding during vaccination was a customary practice, and the control group centres where this practice was not habitual.
In the case of older children, the control group was simply not assigned any particular distraction measure, and was managed according to customary practice in the corresponding primary care centre.
Measurement instrumentsWe used 2 assessment scales (depending on age) and measure the duration of crying in children under 2 years.
- 1
The Neonatal Infant Pain Scale (NIPS).7 The NIPS was developed to assess pain in newborns, but there is a version adapted for use in preverbal children. This scale assesses facial expression, crying, breathing pattern and arm and leg movements. The possible score ranges from 0 (no pain) to 6 points (maximum pain). It can be used in infants aged less than 1 year (Table 1).
- 2
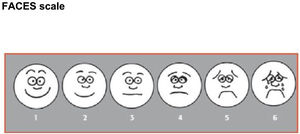
FACES scale: composed by a given number of faces (5–10, depending on the scale) with expressions of gradually increasing discomfort (from a face without pain to a crying face with a grimace of severe pain). Each face is assigned a given score. The scale is applied by asking patients to identify the face with the expression that most closely matches the pain they are experiencing. Scores in this scale range from 1 to 6 points (minimum-maximum). We used the Wong-Baker FACES pain rating scale8 with 6 faces.
- 3
Duration of crying. It was assessed following the method proposed in the study by Nieto García et al.,9 measuring the duration in seconds from the moment crying started following administration of the first shot until the patient had been quiet for a consecutive 5 s, subtracting these 5 s from the counts (maximum time: 3 min).
The primary outcome was pain during vaccination, assessed differently based on age:
- 1
In children under 2 years, 3 approaches were used:
- a)
Assessment of pain with the NIPS.
- b)
Duration of crying.
- c)
Assessment of pain by parents/guardians using the Wong-Baker FACES scale.
- a)
- 2
In children older than 2 years, a visual scale was used: the Wong-Baker FACES scale, for assessment by both children and accompanying parents/guardians.
We estimated that a sample size of 30 participants per group (60 in total) would allow detection of a mean difference in pain of 1.5 points between the intervention and control groups using the Student t-test with a power of 80%. For this calculation, we assumed a standard deviation of 1.7510 for a significance level of 0.05 (bilateral tests). We established that both intervention and control groups would have the same number of patients under and over 2 years of age (50%). The sample size was calculated with the software nQuery version 7.
Statistical analysisWe have described categorical variables as absolute frequencies and percentages, and analysed differences between groups with the chi square test. We described crying and pain scale results using the mean and standard deviation (SD) as well as the median and interquartile range (IQR). In the case of means, comparisons were made with the Student t test, and in the case of medians, with the nonparametric Wilcoxon test. We assessed differences in pain for the overall sample and in the 2 age groups in order to explore potential differences in behaviour based on age (<2 years vs >2 years). We also analysed the correlation between pain assessments conducted by parents/guardians, the paediatric care team and, if applicable, the child. To this end, we calculated the Pearson correlation coefficient with the corresponding 95% confidence interval (CI).
Ethical considerationsThe study was approved by the Ethics Committee of Research with Medicines of the Basque Country in the meeting held on 19/12/2018 (documented in Minute 12/2018).
ResultsThe study included 125 children (60 in the intervention group and 65 in the control group). Table 2 summarises the descriptive characteristics of the total sample and the differences between the intervention and control groups.
Descriptive characteristics of the sample and differences between intervention and control groups.
| Total | Intervention group | Control group | P | |
|---|---|---|---|---|
| N | 125 | 60 (48%) | 65 (52%) | |
| Age | .852 | |||
| 2 months | 44 (35.2%) | 21 (35%) | 23 (35.4%) | |
| 11 months | 21 (16.8%) | 9 (15%) | 12 (18.5%) | |
| 4 years | 60 (48%) | 30 (50%) | 30 (46.2%) | |
| Sex | .672 | |||
| Male | 59 (47.2%) | 30 (50%) | 29 (44.6%) | |
| Female | 66 (52.8%) | 30 (50%) | 36 (55.4%) |
In the analysis of the overall sample (Table 3), we found a significant difference between the intervention and control groups in the assessment by the paediatric care professional (vaccination at 2 months and 11 months), and in the pain perceived by children (vaccination at 4 years). This was consistent with the assessment by parents/guardians (Table 3).
Differences between intervention and control groups in the perceived pain. Assessment by the parent or guardian in every case, and by the patient (age > 2 years) or paediatrician (age < 2 years).
| Total | MD | Intervention group | Control group | P | |
|---|---|---|---|---|---|
| N | 125 | 60 | 65 | ||
| Parent/guardian rating | 3.9 ± 1.5 | 10 | 3.4 (1.3) | 4.5 ± 1.5 | < .001 |
| Median (IQR) | 4 (3−5) | 3 (2−4) | 5 (4−6) | < .001 | |
| Patient/paediatrician rating | 4.2 ± 1.5 | 2 | 3.7 (1.5) | 4.7 ± 1.3 | < .001 |
| Median (IQR) | 5 (3−5) | 4 (2−5) | 5 (4−6) | < .001 |
Results expressed as mean ± standard deviation. The P-value refers to the difference between groups.
IQR, interquartile range, MD, missing data.
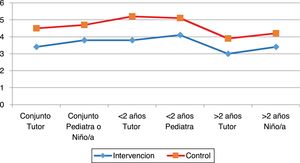
In the analysis by age group, we also found significant differences both for children under 2 years (Table 4) and over 2 (Table 5). The only discordant finding was that the duration of crying in children aged less than 2 years was longer in the intervention group compared to the control group (Table 4, Fig. 1).
Differences between the intervention and control groups in the perceived pain and duration of crying in children aged less than 2 years (2-11 months).
| Total | MD | Intervention group | Control group | P | |
|---|---|---|---|---|---|
| N | 65 | 30 (46.2%) | 35 (53.8%) | ||
| Parent/guardian rating | 4.4 ± 1.2 | 10 | 3.8 ± 1.1 | 5.2 ± 0.7 | < .001 |
| Median (IQR) | 5 (4−5) | 4 (3−4) | 5 (5−6) | < .001 | |
| Patient/paediatrician rating | 4.7 ± 1.2 | 0 | 4.1 ± 1.3 | 5.1 ± 0.8 | < .001 |
| Median (IQR) | 5 (4−5) | 4.5 (4−5) | 5 (4−6) | .001 | |
| Crying | 77.8 ± 55.9 | 5 | 89.4 ± 55.6 | 67 ± 54.8 | .122 |
| Median (IQR) | 58 (40−102.5) | 80 (48−121) | 45 (40−62.5) | .034 |
Results expressed as mean ± standard deviation. The P-value refers to the difference between groups.
IQR, interquartile range, MD, missing data.
Differences in perceived pain in children aged more than 2 years (4 years) between the intervention and control groups.
| Total | MD | Intervention group | Control group | P | |
|---|---|---|---|---|---|
| N | 60 | 30 (50%) | 30 (50%) | ||
| Parent/guardian rating | 3.5 ± 1.6 | 0 | 3 ± 1.4 | 3.9 ± 1.7 | .018 |
| Median (IQR) | 3 (2−5) | 3 (2−4) | 4.5 (3−5) | .017 | |
| Patient/paediatrician rating | 3.7 ± 1.7 | 2 | 3.3 ± 1.7 | 4.2 ± 1.6 | .042 |
| Median (IQR) | 4 (2−5) | 3 (2−4.8) | 4.5 (3−6) | .044 |
Results expressed as mean ± standard deviation. The P-value refers to the difference between groups.
IQR, interquartile range, MD, missing data.
Fig. 2 shows that there were differences between the intervention and control groups in all outcomes, both in the overall analysis or in the analysis by age or rater.
Lastly, we assessed the correlation between the scores given by the family compared to those given by the patient or the paediatrician. Table 6 presents the obtained correlation coefficients with the corresponding 95% CIs. We found that the assessment of the family was correlated to the score given by paediatric providers (in children younger than 2 years) or the children themselves (in children older than 2 years).
Correlation between the scores given by parents or guardians and the scores given by paediatricians (in patients aged < 2 years) or children themselves (in patients aged > 2 years).
| Group analysed | Coefficient (95% CI) |
|---|---|
| Total sample | 0.7 (0.59−0.78) |
| Age < 2 years | 0.67 (0.51−0.80) |
| Age > 2 years | 0.66 (0.49−0.79) |
CI, confidence interval.
The factors analysed in several previous studies in relation to the pain produced in children by vaccination include the number of vaccines administered and their order. The scales used to assess pain have also differed based on age. We will address these two points and the analysis of our results in this discussion.
When it comes to pain produced by vaccination, the number of administered vaccines has an impact on the efficacy of the measures used to reduce pain during vaccination. In a study conducted in Valencia, Spain, in children aged 2, 4 and 6 months compared 3 nonpharmacological pain relief interventions: non-nutritive sucking (pacifier/binky), breastfeeding and administration of a 50% dextrose solution. Breastfeeding achieved a significant reduction in the crying scale at 2 and 6 months, but not at 4 months. The authors concluded that the number of administered vaccines in each vaccination episode was important in relation to pain reduction. Since 2 vaccines are administered at age 2 months, 1 at age 6 months and 3 at age 4 months, they concluded that breastfeeding is effective for pain relief when one or two vaccines are administered, but that the reduction in pain could not be observed with the simultaneous administration of 3 vaccines.9
Given the observed variation in results based on the number of vaccines administered at a single time, for the groups to be comparable in our study, we performed the intervention at the timepoints when children received 2 vaccines in the 2019 routine vaccination schedule of the Basque Country.6 At ages 2 and 11 months, infants receive the hexavalent and pneumococcal vaccines, and at 4 years, the MMR and the varicella vaccines. The order of administration at ages 2 and 11 months was first the hexavalent vaccine and then the pneumococcal vaccine, as this order reduces the pain after the injection. When 2 vaccines are administered at a single time, it is recommended that the least painful be administered first.11,12
Different methods are available to assess pain in children, based on behavioural assessment through observation and obtaining subjective information from the patient.
Behavioural assessment through observation (agitation, crying, gestures associated with pain) or behavioural scales is used in children aged less than 3 years. There are several scales, but they are all similar as regards the parameters to be assessed and their scoring. One is the Face, Legs, Activity, Cry, Consolability (FLACC) scale, named after the 5 behavioural aspects it assesses, or the revised FLACC.13 Another scale that was developed in Spain assesses changes in behaviour and physiological parameters associated with acute stress caused by postoperative pain, known as LLANTO (crying) scale.14 It assesses 5 parameters rated on a 0–2 point scale, so that the total score can range from a minimum of 0 (minimum pain/no pain) and a maximum of 10 (maximum possible pain). This scale allows differentiation between 3 levels of pain: mild (score of 1–3 points), moderate (4−6 points) and intense (7–10 points). A scale widely used in paediatric care for newborns and infants is the NIPS,7 used in our study. The NIPS scale assesses 6 aspects, but the last one concerns the level of arousal. This is an aspect that can be relevant for the assessment of acute pain, but not in the case of pain caused by vaccination, as it never alters with it, which is why we omitted it from the assessment.
Obtaining subjective information from the patient requires the patient to be old enough to understand and provide answers. Before age 3 years, these self-report measures are not reliable, between ages 3 and 7 years, it is possible to use faces scales; and from age 7 years, quantitative scales and the visual analogue scale can be used.15 The use of numerical scales requires the patient to be able to count and understand the meaning of the numbers, skills that usually develop around age 7 or 8 years. The visual analogue scale is a continuous horizontal line delimited by 2 vertical lines that represent the experience of pain. The patient marks on the line the intensity of the experienced pain relative to the two extremes, and this scale can also be used from age 7 years. Since the intervention took place at 4 years, we used the Wong-Baker FACES scale for the older children in our study.8 To facilitate the analysis, we gave the same scales to the parents/guardians to assess the pain observed in the child.
The efficacy of breastfeeding for pain relief in vaccination is endorsed by the medical literature.10,16 In our study, crying was the only parameter on which breastfeeding did not prove effective. This parameter is not assessed as frequently, but there have been other instances in which favourable results were not found. The study by Nieto García et al. did not find significant differences in this parameter between the intervention and control groups.9 In older patients, distraction measures have achieved positive results. This has been confirmed by a systematic review17 and a Cochrane review.18 The distraction measure used in our study was very simple, we provided children with a party horn and instructed them to blow. Some studies have used robots19 and others have used screens.20 The device used in our study is an unsophisticated traditional toy, as we intentionally avoided the use of a mobile phone, as it is not advisable for primary care centres to promote their use due to the deleterious effects it has on children. The American Academy of Pediatrics recommends avoiding exposure to screens completely in children under age 2 years and ensuring controlled and adequate use of screens thereafter.21 In other instances, thermomechanical stimulation devices like the Buzzy have been used to reduce pain with statistically significant results.22,23 This system, used to deliver cold and vibrations to the skin prior to vaccination, shares its nonpharmacological nature with distraction measures, although its efficacy is associated with the analgesic properties of the technique.
The novelty in our study was the inclusion of pain assessment by the legal guardians of the child using a scale to rate the pain observed in the child during vaccination. Correlation coefficient values range from −1 to +1. Values greater than 0 indicate a positive correlation. The strength of the correlation can be moderate (0.4−0.6) and high (0.7−0.9). In our findings, the correlations between the assessments of the parents/guardians and the patients ranged between 0.66 and 0.7, so we considered the correlation between both assessments moderate to high.
FundingThis study was funded as a Bottom UP project in the framework of the 2019 Programme Contract of the provincial delegation of Gipuzkoa of the Department of Health of the Basque Country.
Conflicts of interestThe authors have no conflicts of interest to disclose.
Ada Maneiro Oteiza (Irun Primary Care Centre).
Itxaropena Jacome Querejeta (Hondarribia Primary Care Centre).
Aitziber Luengo Etxebeste (Irun Primary Care Centre).
María Iturria Leiza (Hondarribia Primary Care Centre).
M. Ángeles Alonso Alonso (Ondarreta Primary Care Centre).
Petra Gómez Pérez (Ordizia Primary Care Centre).
Nuria Gañan Iglesias (Pasaia San Pedro Primary Care Centre).
María Teresa Arrospide Arrospide (Beasain Primary Care Centre).
Maider Mateo Abad (Primary Care Research Unit-Gipuzkoa Health Area).
Appendix A presents the members of the Primary Care Research Group of Gipuzkoa.
Please cite this article as: Gorrotxategi Gorrotxategi P, Zabaleta Rueda A, Urberuaga Pascual A, Aizpurua Galdeano P, Juaristi Irureta S, Larrea Tamayo E, et al. Analgesia no farmacológica en la vacunación. Valoración de pediatras, pacientes y tutores. An Pediatr (Barc). 2022;97:199–205.
Presented as an oral communication at the 17th Pediatric Update Congress of the Spanish Association of Primary Care Pediatrics. Madrid, February 13–15, 2020.