There are few studies on effectiveness and safety of bisphosphonate therapy in secondary osteoporosis in children. The aim of this research was to analyse effectiveness and safety of bisphosphonates in secondary osteoporosis in children.
Patients and methodsMulticentre retrospective study in patients younger than 18 suffering from secondary osteoporosis and who have received bisphosphonates. Clinical data were recorded. Bone mineral density (BMD) was assessed in terms of BMD Z-score in lumbar spine (ZBMDls) measured by dual-energy X-ray absorptiometry (DXA). Effectiveness was valued at changes in ZBMDls one and two years after the onset of bisphosphonates and at the decrese in the number of fractures a year. Adverse events reported were recorded. Descriptive and bivariant analysis was performed.
Results32 patients were recruited. ZBMDls increased one year after the onset of treatment ((−2.46 ± 0.96) vs. (−1.54 ± 1.38); p < .001). Fractures a year dicreased significantly (1 (1–2) vs. 0 (0–0.61); p < .001). ZBMDls increase was higher in patients who were able to walk (1.88 ± 0.72 vs. 0.55 ± 0.82; p = .07) and correlated positively with body mass index (BMI)- for- age percentile (rho: 0.564; p < .001). The decrease in the number of fractures a year was higher in patients with lower initial fracture rate (rho: −0,47; p = .006) and with higher initial ZBMDls (rho: −0.47; p = .07). 10 adverse events were reported in 7 patients (22%), all of them intravenous bisphosphonates related. No association was found between adverse events and studied variables.
ConclusionsBisphosphonates are effective in secondary osteoporosis in children. Response seems to be better in patients who are able to walk, well-nourished and in the early stages of the disease. Adverse events were frequent but mild.
Los estudios sobre efectividad y seguridad de los bisfosfonatos en osteoporosis infantil secundaria (OIS) son escasos. El objetivo fue analizar efectividad y seguridad de los bisfosfonatos en OIS.
Pacientes y métodosEstudio multicéntrico retrospectivo en <18 años afectos de OIS tratados con bisfosfonatos. Se recogieron variables clínicas. Se valoró densidad mineral ósea (DMO) mediante el Z-score de DMO en columna lumbar (ZDMOcl) medido por absorciometría de rayos X de doble energía (DXA). Valoramos efectividad en función del cambio del ZDMOcl al año y a los dos años de su inicio y del descenso del número de fracturas/año. Los eventos adversos reportados fueron recogidos. Se realizó análisis descriptivo y bivariante.
ResultadosSe reclutaron 32 pacientes. El ZDMOcl se incrementó al año del inicio del tratamiento ((−2,46 ± 0,96) vs. (−1,54 ± 1,38); p < 0,001). El número de fracturas/año disminuyó significativamente (1 (1–2) vs. 0 (0–0,61); p < 0,001). El cambio en el ZDMOcl fue mayor en los pacientes deambulantes (1,88 ± 0,72 vs. 0,55 ± 0,82; p = 0,07) y se correlacionó positivamente con el percentil del IMC (rho: 0,564; p < 0,001). El descenso del número de fracturas/año fue mayor en los pacientes con menor tasa inicial de fracturas (rho: −0,47; p = 0,006) y cuanto mayor era el Z-score inicial (rho: −0,47; p = 0,07). Se reportaron 10 eventos adversos leves en 7 pacientes (22%), todos con bisfosfonatos intravenosos. No se halló relación entre eventos adversos y las variables estudiadas.
ConclusionesLos bisfosfonatos son efectivos en OIS. La respuesta parece ser mejor en pacientes deambulantes, bien nutridos y en estadios precoces de la enfermedad. Resultan seguros, siendo los efectos adversos leves, aunque frecuentes.
Secondary osteoporosis is an increasingly prevalent disease in paediatric practice due to the increase in life expectancy of chronically ill children and the use of osteotoxic treatments, among other factors.1 It is characterised by a decrease in bone mass and changes in bone microarchitecture that lead to increased bone fragility and therefore a higher risk of fracture.2
The management of secondary juvenile osteoporosis should be multidisciplinary, involving rehabilitation specialists, nutritionists and endocrinologists, among others.1
A key aspect in fighting this disease is the modification of risk factors and supplementation with calcium and vitamin D in patients with insufficient dietary intakes. These measures may be effective in mild cases, but often turn out to be insufficient.1 Therefore, and given the promising results observed in the management of osteogenesis imperfecta and osteoporosis in adults, bisphosphonates are being used with increasing frequency for treatment of secondary juvenile osteoporosis.3
Bisphosphonates are synthetic analogues of pyrophosphate that accumulate in bone. In the first phase of bone remodelling, they are released and taken up by osteoclasts, which impairs their ability to form the ruffled border, adhere to the bone surface and produce the protons and lysosomal enzymes required to proceed with bone resorption.4 They concentrate selectively in the skeleton, primarily at sites of increased bone remodelling, reducing bone resorption, increasing bone mineral density (BMD) and maintaining or even improving structural and material properties of bone under normal conditions.5
They are hydrophilic drugs with a poor absorption by the gastrointestinal tract (<1%) and a high volume of distribution, and are excreted in the urine, so their dose needs to be adjusted based on the glomerular filtration rate. Their elimination from bone tissue is very slow, and they remain in the body for years after administration.5 This, combined with the scarcity of studies assessing the long-term safety of bisphosphonates in children and evidence of teratogenic effects in rat models, has restricted their use in the paediatric age group to the context of paediatric clinical trials or cases of severe disease in which bone fragility had a significant impact on the patient’s quality of life.
The widespread use of bisphosphonates in the management of osteogenesis imperfecta has produced very promising results in terms of the short-, medium- and long-term safety in children,6 which has resulted in the recommendation to contemplate their use in patients with SJO in different consensus guidelines,3,7 especially in cases with a low potential for recovery due to the stage of pubertal development, the persistence of risk factors, etc. However, few studies have assessed the efficacy and safety of bisphosphonates for management of secondary juvenile osteoporosis.8
ObjectiveThe aim of the study was to assess the effectiveness and safety of bisphosphonates for treatment of secondary juvenile osteoporosis (SJO).
MethodsWe conducted a multicentre retrospective longitudinal study in patients aged less than 18 years with a diagnosis of SJO based on the 2019 International Society for Clinical Densitometry (ISCD) criteria9 and treated with bisphosphonates who had undergone at least 2 lumbar bone density scans by dual-energy X-ray absorptiometry (DXA), one before treatment initiation and another approximately 1 year after.
After obtaining informed consent, we collected demographic, anthropometric, clinical and treatment data. We assessed BMD by means of the z-score of the lumbar spine BMD (LS-BMDz) measured by DXA and adjusted for height using the formula proposed by Zemel et al.10 if the height was equal to or less than the third percentile.
We assessed the effectiveness of the treatment based on the change in LS-BMDz 1 year and 2 years after initiation of treatment and the reduction in the number of fractures per year, compared by means of the paired sample t-test. We calculated the incidence of fractures before treatment initiation as the number of long-bone and vertebral fractures that the patient experienced prior to initiation of treatment with bisphosphonates divided by the number of years elapsed from the first fracture to treatment initiation. Likewise, we calculated the incidence of fractures after initiation of treatment with bisphosphonates as the number of long-bone and vertebral fractures that the patient experienced from initiation of antiresorptive therapy divided by the number of years elapsed between treatment initiation and inclusion in the study. We collected information on every documented adverse event.
The statistical analysis started with a descriptive summary of the data, followed by a bivariate analysis to identify factors associated with the effectiveness and safety of bisphosphonates. The changes in BMD z-scores and in the incidence of fractures did not follow a normal distribution, so we used the Mann–Whitney U test to compare qualitative data and calculate the Spearman rho coefficient to compare quantitative data. We set the level of statistical significance at 0.05.
The study protocol adhered to the principles of the Declaration of Helsinki and current international law on human rights, biomedicine and the protection of personal data, and was approved by the Research Ethics Committee of the Hospital Regional Universitario de Málaga. We safeguarded the confidentiality of the data, which were anonymised.
ResultsThe study included 32 patients managed in 6 hospitals. Table 1 summarises the characteristics of the patients. In every patient, the dosage of bisphosphonates was established based on recommendations for management of SJO7 (Table 2).
Demographic, anthropometric, clinical and treatment.
| Demographic and anthropometric characteristics of the patients (N = 32) | |
|---|---|
| Sex (male), n (%) | 21 (65.6%) |
| Age (years), mean ± SD | 10.6 ± 3.55 |
| Height z, mean ± SD | –1.95 ± 1.92 |
| Weight z, mean ± SD | 0.09 ± 1.74 |
| BMI z, median (IQR) | –0.99 (–1.39 to 0.00) |
| Clinical characteristics (N = 32) | |
|---|---|
| Aetiology of secondary osteoporosis, n (%) | |
| Neurologic | 19 (59.4%) |
| Haematological | 6 (18.8%) |
| Other | 7 (21.9%) |
| Ability to stand, n (%) | 20 (62.5%) |
| Ability to walk, n (%) | 14 (43.8%) |
| Pre-treatment fractures, median (IQR) | 1 (1–2) |
| Presence of vertebral fractures (yes), n (%) | 12 (38.7%) |
| Pre-treatment LS-BMDza, mean ± SD | –2.46 ± 0.96 |
| Age at first fracture (years), mean ± SD | 8.9 ± 3.5 |
| Treatments received (N = 32) | |
|---|---|
| Systemic glucocorticoids, n (%) | 8 (25%) |
| Anticonvulsants, n (%) | 11 (34.4%) |
| Vitamin D3 supplements, n (%) | 23 (71.9%) |
| Physical therapy, n (%) | 20 (62.5%) |
| Characteristics of bisphosphonate treatment (N = 32) | |
|---|---|
| Route of administration (IV), n (%) | 25 (78.1%) |
| Type of bisphosphonate, n (%) | |
| Pamidronate | 8 (25%) |
| Aledronate | 5 (15.6%) |
| Zoledronate | 17 (53.1%) |
| Risedronate | 2 (6.3%) |
| Duration of treatment (months), median (IQR) | 21.5 (14–24) |
| Treatment discontinuation, n (%) | 21 (65.6%) |
| Reason for treatment discontinuation, n (%) | |
| LS-BMDza > –2 and absence of fractures for a year | 13 (40.6%) |
| Absence of fractures | 3 (9.4%) |
| Deathb | 2 (6.3%) |
| Other | 3 (9.4%) |
| Adverse events, n (%) | |
| Flu-like illness | 6 (18.9%) |
| Asymptomatic hypocalcaemia | 4 (12.5%) |
BMI, body mass index; IQR, interquartile range; IV, intravenous; LS-BMDz: lumbar spine bone mineral density z-score; SD, standard deviation.
Dosage of bisphosphonates in the sample.
| Drug | Route of administration | Dose |
|---|---|---|
| Pamidronate | Intravenous (diluted in 100–250 mL normal saline, given over 3–4 h) | <1 year: 0.5 mg/kg every 2 months |
| 1–2 years: 0.25–0.5 mg/kg/day 3 consecutive days every 3 months | ||
| 2–3 years: 0.375–0.75 mg/kg/day 3 consecutive days every 3 months | ||
| >3 years: 0.5–1 mg/kg/day 3 consecutive days every 4 months | ||
| Maximum dose: 60 mg/dose and 11.5 mg/kg/year | ||
| Zoledronate | Intravenous (diluted in 50 mL normal saline, given over 1 h) | 0.025–0.05 mg/kg every 6–12 months |
| Maximum dose: 4 mg | ||
| Alendronate | Oral | <40 kg: 35 mg/week |
| >40 kg: 70 mg/week | ||
| Risedronate | Oral | <40 kg: 15 mg/week |
| >40 kg: 30 mg/week |
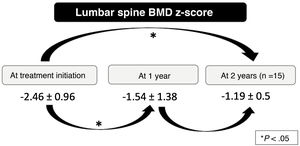
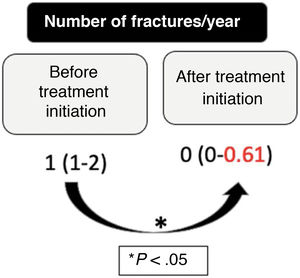
We found a significant increase in the LS-BMDz at 1 year (mean ± standard deviation [SD]: –2.46 ± 0.96 vs –1.54 ± 1.38; P < .001) and at 2 years (mean ± SD: –2.46 ± 0.96 vs –1.19 ± 0.5; P = .001) after treatment initiation, although the differences between the 1-year and 2-year LS-BMDz values were not significant (Fig. 1). The number of fractures per year also decreased significantly after treatment initiation (median [interquartile range]: 1 [1–2] vs 0 [0–0.61]; P < .001), with a mean percentage reduction in fractures of 63.75% (SD, 29.60%) (Fig. 2).
The magnitude of the change in LS-BMDz at 1 year of treatment was greater in patients who were able to walk (mean ± SD: 1.88 ± 0.72 vs 0.55 ± 0.82; P = .07) and was positively correlated to the body-mass index (BMI) percentile at treatment initiation (rho, 0.564; P < .001) (Table 3).
Changes in the lumbar spine mineral bone density z-score at 1 year of treatment based on variables under study.
| Qualitative variables | Change in LS-BMDza | P |
|---|---|---|
| Sex | ||
| Male, median (IQR) | 0.7 (0.38 to 1.24) | .658 |
| Female, median (IQR) | 0.3 (0.00 to 1.60) | |
| Physical therapy | ||
| Yes, median (IQR) | 0.55 (0.00 to 0.85) | .098 |
| No, median (IQR) | 0.85 (0.63 to 1.54) | |
| Vitamin D supplementation | ||
| Yes, median (IQR) | 0.70 (0.30 to 1.37) | .363 |
| No, median (IQR) | 0.70 (–0.05 to 1.73) | |
| Systemic glucocorticoids | ||
| Yes, median (IQR) | 0.75 (0.31–1.25) | .131 |
| No, median (IQR) | 0.70 (0.08 to 1.4) | |
| Antiepileptic drugs | ||
| Yes, median (IQR) | 0.4 (–0.1 to 0.9) | .247 |
| No, median (IQR) | 0.7 (0.33 to 1.55) | |
| Able to stand | ||
| Yes, median (IQR) | 0.76 (0.31 to 1.47) | .089 |
| No, median (IQR) | 0.65 (0.00 to 0.85) | |
| Able to walk | ||
| Yes, median (IQR) | 0.95 (0.64 to 1.37) | .007 |
| No, median (IQR) | 0.65 (0.00–0.92) | |
| Presence of vertebral fractures | ||
| Yes, median (IQR) | 0.33 (–0.08 to 1.25) | .678 |
| No, median (IQR) | 0.70 (0.40 to 1.10) | |
| Bisphosphonate route of administration | ||
| IV, median (IQR) | 0.70 (0.15 to 0.90) | .671 |
| Oral, median (IQR) | 1.37 (0.30 to 1.60) | |
| Discontinuation of treatment | ||
| Yes, median (IQR) | 0.8 (0.30 to 1.55) | .155 |
| No, median (IQR) | 0.4 (–0.1 to 0.71) | |
| Adverse events | ||
| Yes, median (IQR) | 0.70 (0.04 to 2.1) | .852 |
| No, median (IQR) | 0.70 (0.00 to 1.24) | |
| Quantitative variables | Spearman rho | P |
|---|---|---|
| Age (years) | –0.219 | .229 |
| Height z-score at treatment initiation | –0.096 | .606 |
| Weight z-score at treatment initiation | –0.359 | .064 |
| BMI z-score at treatment initiation | 0.587 | <.001 |
| LS-BMDz at treatment initiationa | –0.182 | .319 |
| Fractures/yearb | 0.235 | .195 |
| Duration of treatment (months) | –0.179 | .326 |
BMI, body mass index; IQR, interquartile range; LS-BMDz, lumbar spine mineral bone density z-score; SD, standard deviation.
The decrease in the number of fractures per year was greater in patients with a lower baseline incidence of fractures (rho, –0.47; P = .006) and with higher z-scores at treatment initiation (rho, 0.525; P = .07) (Table 4).
Decrease in fractures per year (%) based on variables under study.
| Qualitative variables | % reduction in fractures | P |
|---|---|---|
| Sex | ||
| Male, median (IQR) | 100 (20.88–100.00) | .870 |
| Female, median (IQR) | 100 (17.31–100.00) | |
| Physical therapy | ||
| Yes, median (IQR) | 100.00 (100.00–100.00) | .157 |
| No, median (IQR) | 91.75 (0.00–100.00) | |
| Vitamin D supplementation | ||
| Yes, median (IQR) | 100.00 (0.00–100.00) | .114 |
| No, median (IQR) | 100.00 (100.00–100.00) | |
| Systemic glucocorticoids | ||
| Yes, median (IQR) | 100.00 (0.00–100.00) | .413 |
| No, median (IQR) | 100.00 (83.50–100.00) | |
| Antiepileptic drugs | ||
| Yes, median (IQR) | 100.00 (23.08–100.00) | .811 |
| No, median (IQR) | 100.00 (0.00–100.00) | |
| Able to stand | ||
| Yes, median (IQR) | 100.00 (0.00–100.00) | .852 |
| No, median (IQR) | 100.00 (23.08–100.00) | |
| Able to walk | ||
| Yes, median (IQR) | 100.00 (0.00–100.00) | .114 |
| No, median (IQR) | 100.00 (100.00–100.00) | |
| Presence of vertebral fractures | ||
| Yes, median (IQR) | 100.00 (0.00–100.00) | .359 |
| No, median (IQR) | 100.00 (80.77–100.00) | |
| Bisphosphonate route of administration | ||
| IV, median (IQR) | 100.00 (0.00–100.00) | .214 |
| Oral, median (IQR) | 100.00 (100.00–100.00) | |
| Discontinuation of treatment | ||
| Yes, median (IQR) | 100.00 (83.50–100.00) | .576 |
| No, median (IQR) | 100.00 (0.00–100.00) | |
| Adverse events | ||
| Yes | 100.00 (100.00–100.00) | .413 |
| No | 100.00 (0.00–100.00) |
| Quantitative variables | Spearman rho | P |
|---|---|---|
| Age (years) | 0.193 | .307 |
| Height z-score at treatment initiation | 0.011 | .957 |
| Weight z-score at treatment initiation | 0.501 | .065 |
| BMI z-score at treatment initiation | 0.124 | .514 |
| LS-BMDz at treatment initiationa | 0.525 | .030 |
| Fractures/yearb | –0.47 | .006 |
| Duration of treatment (months) | 0.252 | .179 |
BMI, body mass index; IQR, interquartile range; LS-BMDz, lumbar spine mineral bone density z-score; SD, standard deviation.
A total of 10 mild adverse events were reported in 7 patients (22%) (flu-like illness in 6 and asymptomatic hypocalcaemia in 4), all of them treated with intravenous bisphosphonates. We did not find an association between adverse events and sex, age, underlying disease, the route of administration of antiresorptive therapy or any of the other variables under study.
Two patients died during treatment from complications of the underlying disease.
DiscussionDespite the recent widespread use of bisphosphonates for treatment of SJO and their recommendation for this indication in different consensus guidelines,3,7 there are few studies on its effectiveness and safety in the management of this disease.8,11–14 Most have assessed its usefulness in the context of specific primary diseases,11,12,14 with infrequent assessment of the reduction in the incidence of fractures as an endpoint,12 and the samples under study tend to be very small.11,14
Our study included patients from 6 hospitals in Spain.
As regards the evidence on effectiveness, while the latest Cochrane review concluded that it was insufficient to recommend treatment of paediatric patients with SJO with bisphosphonates,15 more recent studies have found an increase in BMD measured by DXA in different groups of paediatric patients,8,11,14,16,17 which is consistent with our data, as we found a statistically significant increase in the LS-BMDz at 1 year of antiresorptive therapy that was maintained at 2 years.
As for the frequency of fragility fractures, few studies have analysed this outcome8,11,14,17 and their results are contradictory. A study conducted by Nasomyont et al did not find differences in the incidence of fractures in patients with primary, secondary and glucocorticoid-induced osteoporosis treated with intravenous bisphosphonates.8 However, up to 20% of the sample did not meet the ISCD criteria for definition of osteoporosis and 12% had not had any fractures prior to initiation of treatment with bisphosphonates. Furthermore, some of the patients had received oral bisphosphonates prior to initiation of intravenous zoledronate or pamidronate, which may have resulted in underestimation of the pre-treatment incidence of fractures. Another study, conducted by Rooney et al, also found no differences in the incidence of fracture, probably because most of the included patients did not meet the ISDC criteria for osteoporosis and the pre-treatment fracture incidence was extremely low.17 In agreement with our results, a study conducted by Moon et al did find a significant reduction in the incidence of fractures in paediatric patients with cerebral palsy and secondary osteoporosis after initiation of treatment with bisphosphonates,14 as did the study conducted by Nasomyont et al in patients with spinal muscular atrophy.11
When it comes to evidence of variables that may have an impact on the effectiveness of bisphosphonates, they have not been studies analysing them in children. However, studies in adults have found that the number of fractures decreases more in female patients and that this reduction is directly correlated to the fracture risk assessment (FRAX) score,18 which is used to assess the risk of fragility fracture based on age, sex, BMI, use of substances like tobacco or alcohol, treatment with glucocorticoids, parental history of hip fracture, previous fragility fractures, presence of diseases strongly associated with osteoporosis and the BMD in the femoral neck.19 The subset of patients that exhibited the highest reduction in fractures corresponded to women with glucocorticoid-induced osteoporosis.18 Interestingly, in our study we found a higher effectiveness of bisphosphonates in patients with a lower risk of fragility fractures and at earlier stages of disease, such as patients still able to walk and with a lower incidence of fractures and higher LS-BMDz at treatment initiation. We did not find differences based on sex or previous treatment with oral glucocorticoids. These differences are probably due to the particularities of bone metabolism in children, in which bone modelling and remodelling are combined in cycle that is essential for bone growth during childhood.20 Hormonal differences between children and adults are also at play, as studies in adults include postmenopausal women. Lastly, contrary to the results reported in adults, we did not find differences based on treatment with glucocorticoids. Possible reasons for this difference are that glucocorticoids are usually prescribed at low doses and for the shortest possible duration in paediatric patients and the greater potential for recovery of bone tissue in children compared to adults. We also ought to highlight that the study only contemplated whether patients had received glucocorticoids at any point in their lives, without considering the dose or duration of treatment.
However, both studies in adults and our own study have found a better response in patients with a greater BMI.18 Given that patients in our study generally had low BMI z-scores (Table 1), this outcome may reflect that an adequate nutritional status has a positive impact on bone mass acquisition. In addition, the stronger mechanical stimuli experienced by bone in patients with a greater BMI21 could promote the effect of bisphosphonates.
Other factors, such as the stage of pubertal development or the underlying disease of the patient, also likely have an impact on the effectiveness of bisphosphonates, but we were unable to address this in our study, as the sample was small and the data collection retrospective.
As concerns safety, the proportion of patients that experience adverse events in studies that assess bisphosphonates for treatment of SJO ranges from 16%16 to 48%,8 depending on the underlying disease and the proportion treated with intravenous bisphosphonates. In our study, 22% of patients experienced adverse events, in every case associated with intravenous bisphosphonates. Other studies have found a greater incidence of adverse events associated with intravenous bisphosphonates compared to oral ones,22 which warrants consideration, given that there seem to be no differences in effectiveness, of whether oral bisphosphonates should not be established as the treatment of choice for SJO. However, it is important to take into account that in both our study and studies by other authors, oral bisphosphonates were only prescribed to patients that were able to swallow a whole tablet and remain upright for 30 min following administration and without risk factors for oesophagitis, the most concerning potential adverse event when bisphosphonates are administered orally. In this group of patients, oral bisphosphonates may be considered the first-line treatment, however, if these requirements are not met, the current recommendation is to administer intravenously. In addition, the adverse events reported with the use of intravenous bisphosphonates have been mild, such as flu-like illness after the first infusion or asymptomatic hypocalcaemia. This, combined with the convenience of the dose schedule for zoledronate, makes this drug be one of the most commonly used in paediatric patients, as reflected in the results of our study.
There are limitations to our study. Due to its retrospective design, we were unable to collect data on some important aspects, such as the dietary calcium intake and variables related to physical activity. In addition, the median duration of follow-up was insufficient to assess the long-term effectiveness and safety of bisphosphonates, and the sample was small. We also did not analyse patient outcomes after treatment discontinuation.
Notwithstanding, we can conclude that bisphosphonates are an effective option for treatment of SJO. The response to treatment seemed better in patients with adequate nutritional status, younger age and in the early stages of disease.
Furthermore, although mild adverse events were relatively frequent, it is reasonable to conclude that these drugs are safe for use in children.
In any case, prospective studies in larger samples are required for a more accurate assessment of the efficacy and safety of bisphosphonates in the management of secondary juvenile osteoporosis.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Galindo Zavala R, Bou-Torrent R, Mir-Perelló C, Martínez Regueira S, Magallares-López B, López-Corbeto M, et al. Efectividad y seguridad de los bisfosfonatos en el tratamiento de la osteoporosisinfantil secundaria. An Pediatr (Barc). 2022;97:190–198.
Previous presentation: the results of the study were presented at the Congress of the Sociedad Española de Radiología Pediátrica (SERPE), held November 21–23, 2019 in Madrid, Spain.