Newborn screening is an important method for the early detection and treatment of congenital defects, and it plays a key role in improving children's health.1 Many countries consider newborn screening an important national public health programme, and screening for certain diseases is widespread. Newborn screening for detection of congenital hypothyroidism (CH) is performed worldwide since the 1970s and has proven useful for the detection of this disease. We conducted a retrospective study in which we analysed the clinical data corresponding to CH screening for the past 13 years, focusing on the distribution of the results, the optimal cut-off points for definition of positive results, possible associated factors, etc.
A signed informed consent form was obtained for screening of each newborn. The dried blood spot (DBS) samples were collected from the infants on 903 filter paper at 72 h post birth. The level of thyroid stimulating hormone (TSH) in the samples was measured by means of time-resolved fluoroimmunoassay (TRFIA). In case of a positive result, serum levels of TSH and free thyroxine (FT4) were measured by electrochemical immunoassay (ECIA).
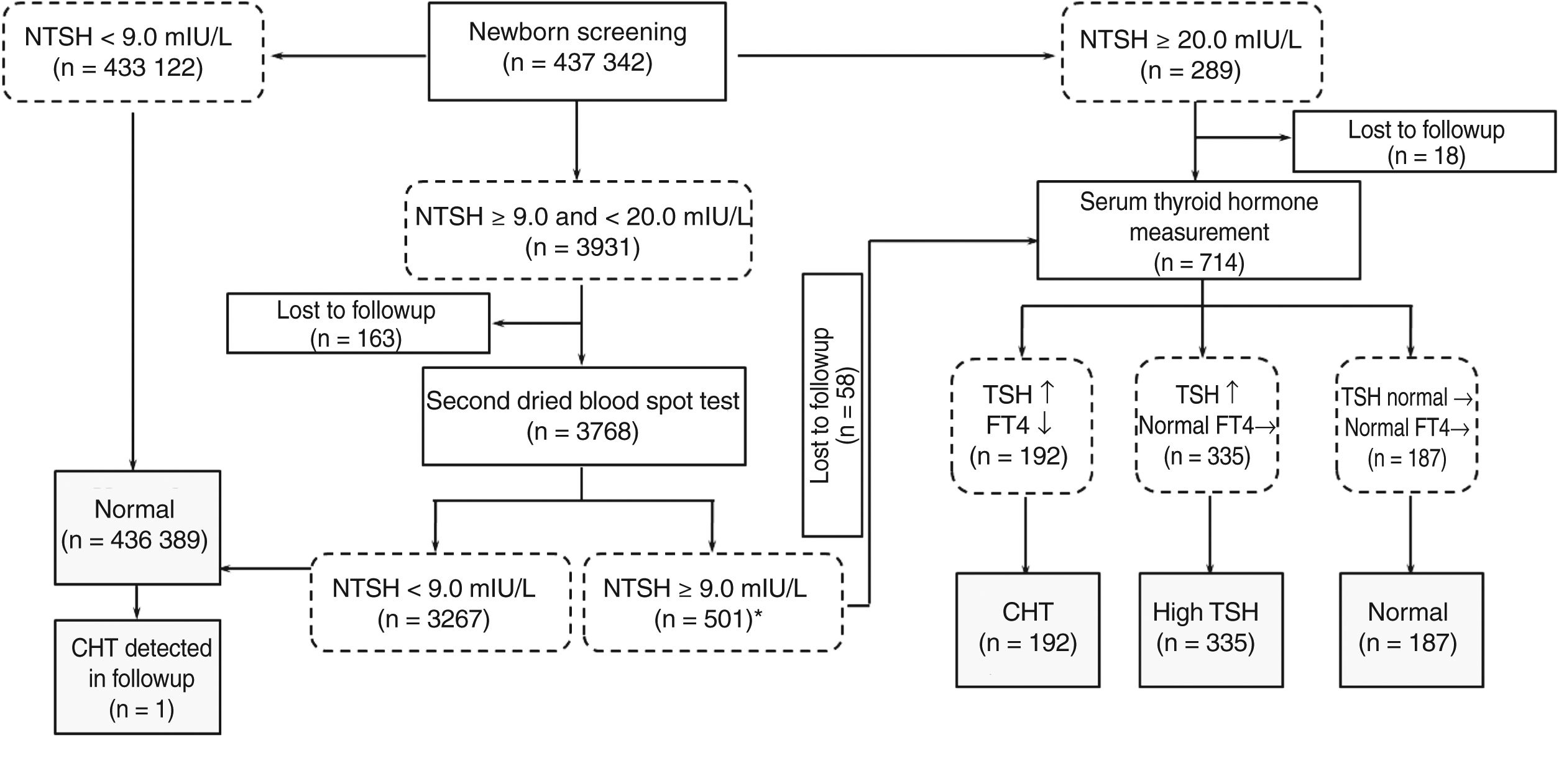
The study includes a total of 437,342 newborn infants for whom consent was given to perform screening. Fig. 1 shows the overall results of the study. A total of 192 infants ultimately received a diagnosis of CH, corresponding to 105 boys and 87 girls. The incidence of CH was of 1 case per 2278 births, which was consistent with the average reported for China.2 At the same time, this diverged slightly from the incidence in other countries.
General results of the study.
Note: *From Jan 2006 to Dec 2009, we used the cutoff as 8.5 mIU/L. During the period, 5 cases of CH and 9 of hyperthyroxinemia were diagnosed with additional recall. Their NTSH were between 8.5 and 9.0.
CH, congenital hypothyroidism; FT4, free thyroxine; NTSH, neonatal thyroid stimulating hormone level; TSH, thyroid stimulating hormone level.
.
In the group of infants with CH, the median level of TSH was 46.10 mIU/L (interquartile range [IQR], 17.90–120.00), compared to 2.39 mIU/L in infants with normal test results. In the subsequent serologic tests, the median serum level of TSH was 75.0 mIU/L (IQR, 75.00–75.00) and the median level of FT4 was 5.14 pmol/L (IQR, 2.83–8.39). The cut-off value used for detection of CH between January 2006 and December 2009 was 8.5 mIU/L. During the period under study, an additional 5 cases of CH were diagnosed, and the serum TSH levels in these infants were 8.58, 8.82, 8.86, 8.87 and 8.95 mIU/L. Screening for CH has been widespread for many years and is proven to be effective. However, a growing number of experts is trying to determine the optimal TSH threshold that should be used to differentiate infants with CH from otherwise healthy newborns and minimise the frequency of false positive results. In order to optimise the TSH threshold, we plotted receiver operating characteristic (ROC) curves and calculated the corresponding areas under the curve (AUCs) with the data of our 430 thousand patients. The AUC for detection of CH was 0.996 applying a cutoff of 9.0 mIU/L. The corresponding sensitivity, specificity, precision, positive predictive value (PPV) and negative predictive value (NPV) were 96.89%, 99.13%, 99.13%, 4.70% and 99.99%, respectively. The optimal cut-off value in our study was 8.58 mIU/L, as it yielded the best combination of sensitivity (99.48%) and specificity (97.70%). Similar studies have been carried out in other countries, corroborating that optimization of the TSH threshold can improve the effectiveness of CH screening.3,4 We also believe that it is necessary to adjust the TSH threshold for local populations through the analysis of retrospective data.
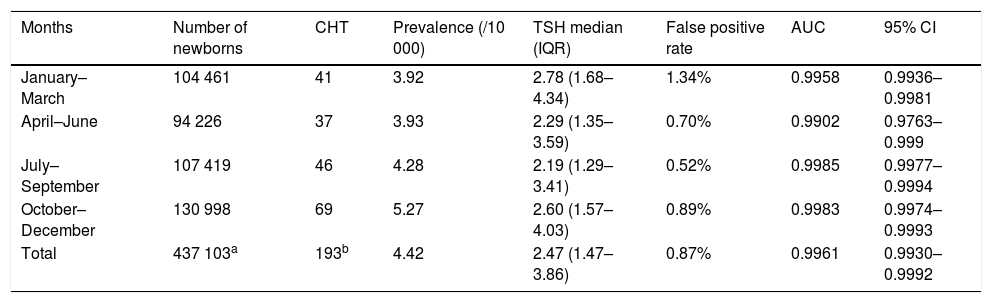
Evidence from various studies suggests that CH is associated with multiple factors. The season of birth season is one of factors that have attracted the attention of researchers. Table 1 showed the data of the association between the screening indicators and the season of birth. We found that the newborn screening programme of CH achieved the best results in the fourth trimester of the year, with an AUC greater than 0.99 and a precision of more than 95%. This was similar to the results reported by Khanjani et al.5 Several authors have identified different factors that may be involved in the association between the prevalence of CH and the season of birth, such as viral infection, differences in climate, living conditions, or the differences in iodine levels that exist between geographical areas.5
Association between screening indicators and season.
| Months | Number of newborns | CHT | Prevalence (/10 000) | TSH median (IQR) | False positive rate | AUC | 95% CI |
|---|---|---|---|---|---|---|---|
| January–March | 104 461 | 41 | 3.92 | 2.78 (1.68– 4.34) | 1.34% | 0.9958 | 0.9936–0.9981 |
| April–June | 94 226 | 37 | 3.93 | 2.29 (1.35– 3.59) | 0.70% | 0.9902 | 0.9763–0.999 |
| July–September | 107 419 | 46 | 4.28 | 2.19 (1.29–3.41) | 0.52% | 0.9985 | 0.9977–0.9994 |
| October–December | 130 998 | 69 | 5.27 | 2.60 (1.57– 4.03) | 0.89% | 0.9983 | 0.9974–0.9993 |
| Total | 437 103a | 193b | 4.42 | 2.47 (1.47– 3.86) | 0.87% | 0.9961 | 0.9930–0.9992 |
AUC, area under the curve; CH, congenital hypothyroidism; CI, confidence interval; IQR, interquartile range; TSH, thyroid-stimulating hormone.
In conclusion, our analysis of a large clinical sample confirmed that the incidence of CH was reliable and the newborn screening programme was effective. However, the yield of screening programmes can still be improved by adjusting the TSH thresholds applied in local screening programmes and correcting for possible confounding factors, such as the season.
FundingThis study was supported by grants from the Key Research and Development Plan Project of Jiangsu Province (BE2017650), the Changzhou Science and Technology Support Project (Social Development CE20175021), and the Jiangsu Mother and Child Health Research Project (F201671).
Please cite this article as: Zhang Y, Yang Y, Mu H, Chen J, Jian J. Cribado neonatal de hipotiroidismo congénito: estudio observacional de 13 años. An Pediatr (Barc). 2020;93:50–52.