Although infection by human papillomavirus (HPV) is mainly considered a sexually transmitted disease, newborns exposed to the virus in the perinatal period can also be infected through mechanisms that are not yet fully understood. The aim of our study was to increase our understanding of neonatal oropharyngeal infection by HPV, trying to establish its frequency, mechanisms of infection and persistence through age 2 years.
Material and methodsWe conducted a prospective, observational and descriptive study in a cohort of neonates born vaginally whose mothers carried HPV in the lower genital tract at the time of delivery. Tests for detection of HPV in amniotic fluid, venous cord blood and oropharyngeal secretions were performed in every neonate, and we conducted microbiological follow-up of infants colonized by HPV up to age 2 years.
ResultsThe prevalence of oropharyngeal colonization at birth was 58.24%. In the 24-month follow-up, the proportions of clearance and persistence of HPV in the oropharynx were 94.34% and 5.66%, respectively.
ConclusionsThe results of this case series suggest that neonatal oropharyngeal colonization by HPV, while frequent in the postpartum period, is usually a self-limited process, and the main mechanism of infection transvaginal intrapartum vertical transmission. Although colonization in most neonates is transient and asymptomatic, the clinical significance of persistent carriage remains unknown.
Aunque la infección por el virus del papiloma humano (VPH) es considerada esencialmente como una enfermedad de transmisión sexual, los recién nacidos expuestos al virus durante el período perinatal pueden también contraer la infección por mecanismos que aún no se conocen con exactitud. Con la presente investigación pretendemos profundizar en el estudio de la infección orofaringea neonatal por VPH, tratando de establecer su frecuencia, mecanismos preponderantes de contagio y persistencia en los dos primeros años de vida.
Material y métodosEstudio observacional, descriptivo y prospectivo de una cohorte de recién nacidos por parto vaginal cuyas madres eran portadoras de VPH en el tracto genital inferior en el momento del parto. Se determinó la presencia del virus en líquido amniótico, sangre venosa de cordón y orofaringe neonatal en todos los casos, manteniendo un seguimiento microbiológico de los neonatos colonizados por VPH hasta los dos años de vida.
ResultadosLa tasa de colonización orofaringea por VPH al nacimiento fue del 58,24%. Para el seguimiento realizado de 24 meses las proporciones de aclaramiento y persistencia viral en orofaringe neonatal fueron 94,34% y 5,66%, respectivamente.
ConclusionesLos resultados de nuestra serie hacen suponer que aunque frecuente en el postparto, la colonización orofaríngea neonatal es un proceso generalmente autolimitado, cuyo principal mecanismo infectivo es la transmisión vertical transvaginal e intraparto. Aunque la mayoría de estas colonizaciones son transitorias y asintomáticas, la transcendencia clínica de los casos de persistencia viral, sigue siendo un enigma.
Infection by human papillomavirus (HPV) is the most frequent sexually transmitted disease in humans.1 Although it usually affects young adults involving the anogenital region, it may also involve other distant anatomical regions, such as the oral cavity, the pharynx or the larynx, and affect any age group.2
For many years, the interest in HPV has chiefly focused on its association with cervical cancer. However, evidence on additional target organs for the pathogenicity of the virus and the progressive increase in certain diseases mediated by HPV, some of which may even occur in infants, has broadened the scope of interest in the field of research on HPV infection.3
Our current knowledge of the causes, frequency and clinical impact of perinatal oropharyngeal infection by HPV is still limited. Our aim was to delve more deeply in the investigation of this pathology.
The main objectives of the study were:
- 1
To establish the prevalence of perinatal oropharyngeal colonization by HPV in neonates born vaginally whose mothers were asymptomatic carriers of HPV in the lower genital tract at the time of delivery.
- 2
To establish the prevalence of persistent oropharyngeal colonization by HPV at ages 12 and 24 months.
- 3
To estimate the proportion of oropharyngeal HPV carriage that may not be attributed to the passage of the foetus by a birth canal colonised by HPV.
To pursue the established objectives, we conducted a prospective observational and descriptive study in a cohort of neonates delivered vaginally whose mothers carried HPV in the genital tract at the time of childbirth.
At the time of delivery, we obtained samples to assess for the presence of HVP in maternal cervicovaginal secretions, amniotic fluid, venous umbilical cord blood and the neonatal oropharynx. Later during the follow-up, infants found to have been colonised by HPV at birth underwent 3 tests for detection of HPV DNA in oropharyngeal secretions. The first one was conducted at 7–10 days post birth, the second at 12 months post birth and the third at 24 months post birth. Thus, we made serial observations through time in the same cohort of colonised patients, with individual follow-up based on the results.
All pregnant women that delivered in our hospital were considered eligible for the study as long as they had a positive result in the hybrid capture HPV test performed in the framework of the cervical cancer screening programme and did not have lesions associated with HPV in the birth canal. Births could be induced electively for obstetric reasons other than premature rupture of membranes, and foetuses had to be delivered vaginally for inclusion in the study.
In the region of Andalusia, Spain, the population-based cervical cancer screening programme includes all women aged 25–65 years.
We excluded deliveries complicated by premature rupture of membranes, pregnant women with immunosuppression and pregnant women in treatment with medication that could modify the viral replication cycle.
The reason why cases of premature rupture of membranes were excluded was to avoid potential biases deriving from undetected prepartum foetal colonization through the ascending transcervical route, and the potential “rinsing effect” that the leaking fluid could have on the HPV present in the maternal lower genital tract.
As regards sample collection, the sample of maternal endocervical secretions was taken during the latent phase of labour, before the rupture of membranes. Samples of amniotic fluid were obtained by artificial rupture of membranes with a sterile amnioscope. Once the newborn had exited the birth canal, a sample of blood was drawn from the umbilical vein after cord clamping (using sterile equipment and following washing of the cord with sterile saline to prevent potential complications). Lastly, oropharyngeal secretion samples were obtained from newborns within minutes of birth, and additional samples were collected 7–10 days, 12 months and 24 months post birth.
When it came to amniotic fluid and venous cord blood samples, the initial fractions of the sample obtained through the needle were discarded to minimise the risk of contamination with maternal secretions.
The rationale for obtaining cord blood samples from the umbilical vein instead of one of the 2 arteries was that inside the uterus, the umbilical vein is the vessel that perfuses the foetus and therefore the ideal site to explore the potential haematogenous transmission of HPV from the mother to the foetus.
The oropharyngeal secretion samples were obtained by firmly rubbing the surface of the tongue, the mucosa of the cheeks, the hard palate and the back of the throat with a single sterile swab.
In relation to sample size calculation, we ought to highlight that the proportion of HPV carriage in pregnant women in our area is unknown. There are no local, regional or even national estimates that would allow inference of its prevalence, so making the assumption that the scarce proportions reported in Europe could be extrapolated to our area,2,5 we estimated that the frequency could be of 200–300 pregnant women per year. For the sample size under study (n=90), obtained by stratified random sampling to obtain a representative sample, we calculated a maximum error of estimation of 8.66% for a 95% confidence level.
Sample processing adhered to the guidelines of the Department of Microbiology of our hospital. Molecular techniques (general primer GP5+/GP6+-mediated polymerase chain reaction [PCR] enzyme immunoassay [EIA] method) were used for microarray-based detection of viral DNA in each of the samples, in addition to identification of the viral serotype (GP5+/GP6+ bio PCR-EIA, Labo Biomedical Products, Rijswijk, Netherlands).
The entire phase of study design and execution was coordinated by a specialist in research methods and statistical analysis who was not part the working group. In addition, the entire study process was overseen and approved by the Research Ethics Committee of our hospital.
ResultsOf the 117 pregnant women potentially eligible for the study, 90 met the inclusion criteria. The 27 cases that were excluded corresponded to 17 pregnant women that underwent a caesarean section and 10 in which data collection was incomplete due to losses to follow-up after birth.
The mean maternal age was 34.91years, and the range was 31–39 years.
At the time of delivery, viral DNA was detected in the cervix of the 90 participants, with identification of 11 cases of multiple infection (defined as the simultaneous presence of 3 or more HPV serotypes). A total of 129 genotypes were detected in the 90 endocervical samples, 9 in the amniotic fluid samples, 6 in the venous cord blood samples and 83 in the neonatal oropharyngeal secretion samples.
The 9 serotypes identified in amniotic fluid corresponded to 7 cases, which means that approximately 7.77% of mothers with lower genital tract HPV carriage also had HPV in the amnion. The 6 genotypes identified in cord blood corresponded to 5 patients.
In every case that HPV was found in the cord blood, it was also detected in the amniotic fluid, with absolute agreement as regards the number and identity of the detected genotypes. The same correspondence was not observed when it came to the detection of maternal endocervical carriage, as in 1 case one of the HPV genotypes detected in both amniotic fluid and venous cord blood was not found in the cervix.
As regards neonatal oropharyngeal colonization by HPV at birth, the virus was detected in 53 of the 91 newborns, which corresponded to a proportion of oropharyngeal colonization of 58.24%. One of the pregnancies in the study was a dichorionic diamniotic twin pregnancy in which both foetuses were colonised by HPV-16.
Table 1 summarises the frequency and distribution of HPV genotypes detected at birth in the different sites.
Frequency of detection of human papillomavirus genotypes by DNA testing in the different sites under study.
| Maternal endocervix | Amniotic fluid | Umbilical cord blood | Neonatal oropharynx | |
|---|---|---|---|---|
| HPV-6 | 31 | 2 | 0 | 22 |
| HPV-11 | 12 | 1 | 1 | 7 |
| HPV-16 | 39 | 2 | 2 | 29 |
| HPV-18 | 17 | 2 | 2 | 13 |
| HPV-31 | 8 | 0 | 0 | 3 |
| HPV-35 | 7 | 1 | 1 | 2 |
| HPV-38 | 5 | 1 | 0 | 2 |
| HPV-52 | 3 | 0 | 0 | 2 |
| Other | 7 | 1 | 0 | 3 |
In the group 53 mother-child dyads in which HPV was detected in the neonatal oropharynx, there was complete agreement as regards the number and identity of identified genotypes in 44 cases. In 8 cases, there were differences between mother and child on account of the absence in the neonatal oropharynx of some of the HPV genotypes detected in the maternal cervix. There was also one case of discordance between mother and child in which HPV-35 was found in the neonatal oropharynx but not in the maternal cervix. In this case, HPV-35 was detected in the neonatal oropharynx, the amniotic fluid and the venous cord blood samples.
In the cases of detection of HPV in oropharyngeal samples in which the virus was also detected in amniotic fluid and umbilical cord samples, there was a perfect match in the number and identity of genotypes detected in each site. However, as we just noted, there was one case in which one of the genotypes detected in the neonatal oropharynx, the amniotic fluid and the venous cord blood could not be found in the maternal cervix.
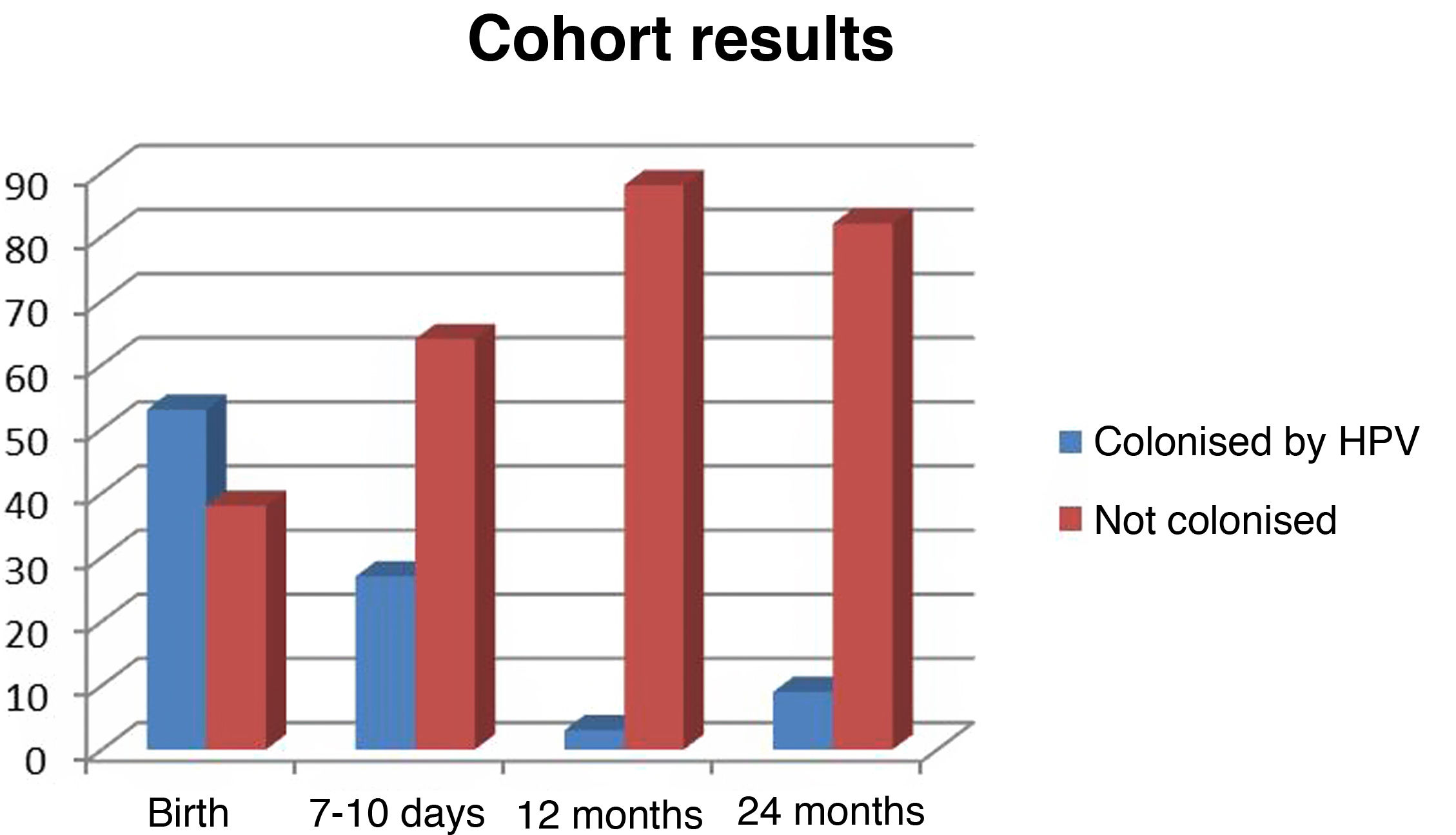
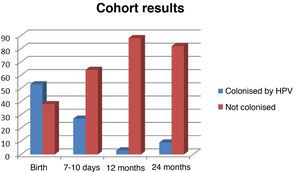
Of the 53 neonates identified as colonised or infected at birth, only 27 (50.94%) were found to continue carrying the virus 7–10 days post birth, and at 1year post birth, HPV carriage was only detected in 3 children (5.66%).
At 12 months post birth, none of the genotypes identified in follow-up testing differed from those identified in the initial observation. There were cases of total or partial clearance of HPV in the nasopharynx, but none in which there was evidence of infection or colonization by additional HPV genotypes postnatally. The group of children with persistent HPV infection at 12 months post birth (n=3) was characterised by colonization of the maternal cervix by 3–5 genotypes in the absence of amniotic or haematogenous spread of HPV and with a predominance of serotype 16 (total of 5 serotype identification: 3 of HPV-16, 1 of HPV-11 and 1 of HPV-6). All were male newborns with HPV-16, of whom 1 was also positive for DNA for serotypes 11 and 6.
At 24 months post birth, DNA testing resulted in 14 genotypes detections in oropharyngeal samples from 9 children. This group included the 3 children that remained positive for HPV with the same genotypes identified at birth at the 12 months timepoint and 6 children with newly diagnosed infections in whom the PCR tests the year before had been negative.
Fig. 1 summarises the evidence on perinatal oropharyngeal colonization obtained during the follow-up.
When we reviewed the health records of the 6 children newly colonised by HPV, we did not find documentation of any care episodes related to dyspnoea, dysphonia, cutaneous or mucosal lesions or any other manifestations suggestive of acute HPV infection.
DiscussionThe findings in this case series suggest that up to 60% of women that carry HPV in the lower genital tract may pass the infection to their offspring during childbirth. Of the total of newborns colonised by HPV, only 6% will develop persistent oropharyngeal infection, but the prognosis of this condition remains uncertain.
While several studies have confirmed that newborns exposed to HPV in the perinatal period may acquire the infection and even develop symptoms,4 the specific mechanisms through which the infection is acquired have yet to be elucidated.
It has been hypothesised that vertical HPV transmission may depend on the passage of the foetus through a contaminated birth canal, transcervical ascending colonization5 or even transplacental passage of the virus.4,6,7 There have also been authors that have proposed the possibility of perinatal infection resulting from periconceptional transmission,8 consumption of breastmilk9 or even skin-to-skin contact with relatives immediately after birth.10
The reported prevalence of neonatal infection by HPV in the offspring of mothers with HPV carriage during pregnancy varies widely, ranging from 4% and 79%.11–22 In a meta-analysis published in 2005, Medeiros et al.23 estimated that it is most plausible that the frequency of perinatal transmission is around 20%. In any case, the natural history of the disease is largely unknown, especially its clinical repercussions.1,4,23 Thus, it is crucial that future research is conducted to determine the factors at play in perinatal transmission and viral persistence. It is also important to elucidate the impact that infection by HPV may have on pregnancy outcomes.
Before delving into the analysis of the results of our case series, it is important that we highlight the considerable limitations of the literature on the subject. The studies conducted to date are relatively scarce, mostly observational and very heterogeneous in methodology, so that it would be difficult to extrapolate the information obtained from any one series to other settings.
When it comes to the factors that increase the risk of perinatal infection by HPV, the only ones that have been found to be unequivocally associated with it is maternal colonization by HPV and the presence of genital warts during pregnancy.4,24 There is also evidence suggesting that a high viral load, maternal coinfection by different HPV serotypes and the mode of delivery could substantially increase the risk of perinatal infection,16,20,22,25–27 but the data on these aspects are contradictory. In any case, both the acquisition of infection and the severity of the resulting disease seem to depend more on other factors, such as ethnicity, the specific human leukocyte antigen class II alleles or the viral genotype.6 A clear indication of this is that caesarean delivery does not prevent the perinatal transmission of HPV.23,28
The main objective of our study was to establish the prevalence of neonatal oropharyngeal colonization by HPV following vaginal delivery in newborns of immunocompetent mothers that carried HPV in the lower genital tract at the time of childbirth. The figures reported in the literature vary widely11–23,28–31 and range between slightly over 5% and nearly 70%.
It is important to consider, on one hand, that detection of HPV in a sample does not necessarily entail infection,3,23 unless the presence of HPV persists through time. On the other, the presence of HPV in the neonatal oropharynx is not univocal and unquestionable evidence of intrapartum transmission.3,10,23,28,32 There are several cases in our series that provide clear illustrations of these considerations.
Our series, with a low proportion of HPV detection in cord blood and amniotic fluid, suggests that the vertical transmission from mother to child mainly, although not exclusively, involves an intrapartum and transvaginal mechanism. At any rate, we cannot rule out the possibility that the HPV colonization detected in each of the cases in the cohort had not occurred before or even after childbirth. In fact, the findings at 24 months post birth in the cohort clearly suggest that a large part of childhood infections by HPV result from postnatal colonization.
As pertains viral persistence in the neonatal oropharynx, based on the data from this case series, we estimate that the prevalence of persistent infection in our region exceeds 5% at age 2 years. It must be taken into account that there is no unanimously accepted definition for the intuitive concept of persistent oropharyngeal infection. Based on what has been established for endocervical infection,1 our group decided to apply a chronological criterion of 24 months for diagnosis of persistent infection.33
An important limitation in the study design was that we assumed that the samples of amniotic fluid and umbilical vein blood were representative. The method used for sample collection does not preclude inadvertent contamination with HPV from maternal lower genital tract secretions, but obtention of samples of higher quality would have required the use of invasive techniques (amniocentesis and cordocentesis), which is absolutely unjustified from an ethical standpoint.
Another methodological limitation of the study is that we only included pregnant women older than 31years. The reason we established this threshold was to set a convenient framework for maternal HPV testing in the cervical cancer screening programme that is currently in place in our region.
One of the questions that remains unanswered is the clinical relevance of neonatal exposure to HPV. It is very likely that a large part of neonatal colonization cases are transient and irrelevant, given the current burden of disease attributable to HPV.34 In our case series, the prevalence of colonization decreased by nearly half in just 10 days and persisted at the 2-year time point in barely 6% of previously colonised individuals, which suggests that a great part of the cases of infection or colonization by HPV are transient.
Lastly, the HPV genotype distribution observed in the series conformed to the epidemiological patterns typically described for the oropharynx and the female lower genital tract in our region.19,34 The most prevalent genotypes are 6, 11, 16 and 18. The potential impact of primary vaccination in the results obtained in our study was low, as only 3 pregnant women had received 1 or more doses of HPV vaccine at the time of testing.
The preventive potential of maternal vaccination against HPV genotypes 6, 11, 16 and 18 is very high, as 100% of persistent infections in our case series involved these serotypes. In fact, at present there is solid evidence supporting vaccination against HPV for prevention of persistent oropharyngeal colonization by this virus.33,34
ConclusionAlthough neonatal oropharyngeal colonization is frequent in the postpartum period, most infections tend to clear relatively quickly, spontaneously and without causing any symptoms. Still, nearly 6% of colonised newborns will develop persistent oropharyngeal infection, which carries an uncertain prognosis.
The findings in our sample suggest that the main, although not sole, mechanism for vertical transmission of HPV in our region is the passage through the birth canal during delivery.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hijona Elósegui JJ, Sánchez Torices MS, Fernández Rísquez AC, Expósito Montes JF, Carballo García AL. La infección orofaríngea neonatal por VPH en nuestro medio. An Pediatr (Barc). 2022;97:112–118.