Hypogammaglobulinemia in the first months after allogeneic hematopoietic stem cell transplantation (HSCT) is common in paediatric patients. During this phase, replacement therapy with human immunoglobulin must be administered parenterally to prevent infections. In some cases, this hypogammaglobulinemia persists over time, which forces further treatment when the patient is usually no longer a carrier of a central line, making them ideal candidates for subcutaneous replacement therapy. There is little published literature describing the use of this method in paediatric patients undergoing HSCT, widely described in replacement treatment in children with primary immunodeficiencies with very good results.
Patients and methodsAn observational, descriptive, longitudinal and retrospective study is carried out. During the years 2008–2019, we evaluated all paediatric patients undergoing HSCT in our center with persistent chronic hypogammaglobulinemia (for over a year). The treatment phase with intravenous immunoglobulin (Privigen®) and the first four years of treatment with subcutaneous immunoglobulin (Hizentra®) are evaluated using a questionnaire.
ResultsDuring the years 2008–2019, 175 patients underwent HSCT, 143 (82%) of whom exceeded three months after transplantation. Three (2%) of them had persistent hypogammaglobulinemia. All three share factors described in the literature involved in immune reconstitution. After analysing the questionnaire, it is observed that switching from intravenous to subcutaneous gammaglobulin has involved a great improvement in their quality of life.
ConclusionsThe origin of chronic hypogammaglobulinemia in our patients shows different factors and cannot be attributed to a single cause. Due to the limited number of patients no conclusions can be drawn at the population level. We have been able to observe that replacement treatment with Hizentra 20% has been as effective as the intravenous administration without evidence of an increase in bacterial infections. Furthermore, it has also led to an improvement in quality of life and increased comfort, as the patients themselves have stated.
La hipogammaglobulinemia en los primeros meses post trasplante alogénico de precursores hematopoyéticos (TPH) es común en pacientes pediátricos. Durante esta fase se debe administrar tratamiento sustitutivo con inmunoglobulina humana por vía parenteral para la prevención de infecciones. En algunos casos, esta hipogammaglobulinemia persiste en el tiempo, lo que obliga a prolongar el tratamiento cuando el paciente ya no suele ser portador de una vía central, por lo que son candidatos ideales para el tratamiento de reemplazo por vía subcutánea. Existe escasa bibliografía publicada que describa el uso de esta vía en pacientes pediátricos sometidos a TPH; que sin embargo está ampliamente descrita y con muy buenos resultados en el tratamiento de reemplazo en los niños con inmunodeficiencias primarias.
Pacientes y MétodosSe realiza un estudio observacional, descriptivo y longitudinal, de carácter retrospectivo. Durante los años 2008–2019 se evalúan a todos los pacientes pediátricos sometidos a TPH en nuestro centro que presentan una hipogammaglobulinemia crónica persistente (superior a un año). Se evalúa la fase de tratamiento con inmunoglobulina endovenosa (Privigen®) y los primeros cuatro años de tratamiento con inmunoglobulina subcutánea (Hizentra®) mediante un cuestionario.
ResultadosDurante los años 2008–2019 se han realizado en nuestro centro 175 trasplantes de precursores hematopoyéticos, de los cuáles 143 (82%) superaron los tres meses post trasplante. De éstos, 3 (2%) presentaron una hipogammaglobulinemia persistente. Los tres comparten factores descritos en la bibliografía involucrados en la reconstitución inmune. Mediante el cuestionario se observa que el cambio de gammaglobulina endovenosa a subcutánea ha supuesto una gran mejora en la calidad de vida de los pacientes.
ConclusionesEl origen de la hipogammaglobulinemia crónica de nuestros pacientes presenta diferentes factores y no se puede atribuir a una única causa. Debido al limitado número de pacientes no es posible establecer conclusiones a nivel poblacional. Hemos podido observar que el tratamiento de reemplazo con Hizentra 20% ha sido igual de eficaz que la vía intravenosa, sin evidenciarse un aumento en las infecciones bacterianas. Además, ha supuesto una mejoría de la calidad de vida y mayor comodidad expresada por los propios pacientes.
Hypogammaglobulinaemia is a frequent complication in the first months after haematopoietic stem cell transplantation (HSCT) in children and young adults1. It is generally defined by serum levels of immunoglobulin G (IgG) of less than 400 mg/dL1 or below the normal range, especially in children aged less than 2 years.
This state results from a delay in B cell recovery and low levels of IgG before transplantation1,2. Multiple factors involved in immune reconstitution have been described in the literature1–7, chief of which are patient age, underlying disease, history of chemotherapy, pretransplant conditioning, use of rituximab8 (anti-CD20), the source of haematopoietic stem cells, T cell depletion, human leukocyte antigen (HLA) matching, serum varicella-zoster virus status, graft versus host disease (GvHD), immunosuppressive therapy, antimicrobial treatment and donor lymphocyte infusion.
While the innate immune system1,2,4 (granulocytes, monocytes and natural killer [NK] cells) tends to recover quickly following transplantation (usually within 3 months), the reconstitution of the acquired immune system (T and B cells) takes longer. Therefore, antibody production cannot usually be detected before 6 months post transplantation. In the case of IgM, recovery may take 3–6 months, while for IgG it tends to take longer, at least 12 months, or more if there are complications such as GvHD, in the context of which full recovery of humoral immunity may never be achieved4.
With the aim of keeping bacterial infections to a minimum and mitigating viral reactivations in the first year post transplantation1,2, administration of human immunoglobulin is recommended to maintain levels above 400 mg/dL, although this treatment always needs to be adjusted to patient age, comorbidities and clinical condition.
Immunoglobulins were first used in patients with primary antibody deficiency, initially administered intramuscularly, in 1980, and subsequently intravenously1. Using either route, patients frequently experienced adverse events such as fever, local pain or headache. In 1991, evidence emerged that subcutaneous immunoglobulin administration was equally effective, well tolerated and associated with fewer adverse events9. Today, a large proportion of patients with primary immunodeficiency, with significant quantitative or qualitative defects in antibody production, receive subcutaneous immunoglobulin replacement therapy due to the pharmacokinetic advantages and fewer adverse events of this route of administration1.
In the immediate post-transplantation phase, immunoglobulin is administered intravenously, as it is meant to be a short-term treatment and patients still have the central venous catheter in place. Although there is evidence that the use of subcutaneous immunoglobulin in the paediatric age group is safe and efficacious10, there is limited experience with its use in post-transplantation paediatric patients1.
The primary objectives of the study were to describe the factors found to be associated with chronic post-transplantation hypogammaglobulinaemia in our patients and describe, by means of a survey, the level of satisfaction and quality of life achieved with at-home subcutaneous immunoglobulin administration. The secondary objective was to assess whether there was a difference in infections with the use of intravenous versus subcutaneous immunoglobulin replacement.
Material and methodsWe conducted a retrospective, longitudinal, observational and descriptive study in our hospital in the 2008–2019 period. We studied paediatric patients with chronic hypogammaglobulinaemia (defined as hypogammaglobulinaemia persisting more than 1 year post HSCT).
In this study, we analysed the initial phase of treatment with intravenous immunoglobulin (Privigen®) and the first 4 years of treatment with subcutaneous immunoglobulin (Hizentra®). The variables under study were the age of the patient at diagnosis and at transplantation, the underlying disease, chemotherapy before transplantation, the source of haematopoietic stem cells, HLA matching, T-lymphocyte depletion, immunosuppressive therapy, presence of GvHD, infections during post-transplantation phase, immunoglobulin levels during replacement therapy and IgG levels before HSCT.
We collected data by reviewing health records and by means of a telephone interview after obtaining informed consent from the parents. In the first phase of the study (retrospective phase), we interviewed the parents on account of the young age of the patients and their lack of reliable recall. In this phase, we assessed the treatment with intravenous immunoglobulin. The second phase (current phase) involved interviewing the patients themselves (along with the parents), as they are currently at an age in which they are aware of their own disease and capable of appraising the treatment with subcutaneous immunoglobulin.
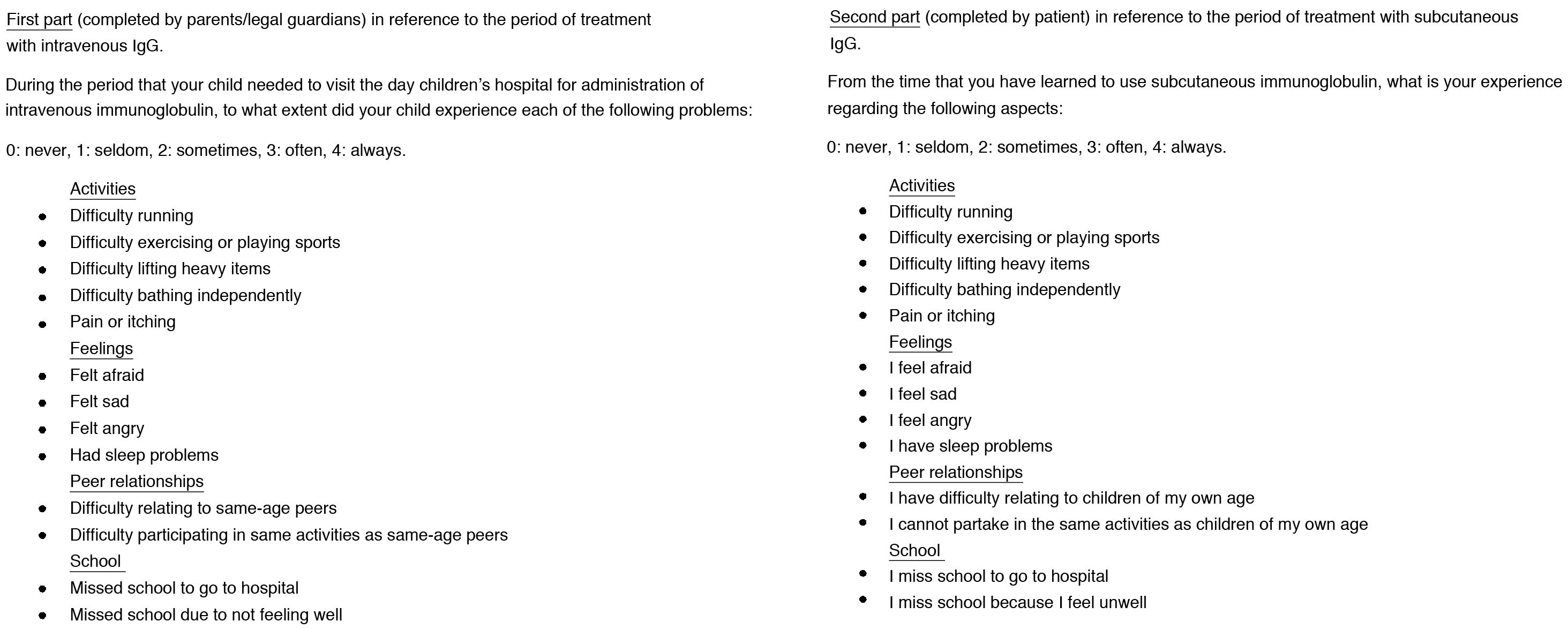
The interviews were conducted with a questionnaire developed specifically for the study (Fig. 1) based on existing paediatric quality of life questionnaires used to assess quality of life in patients with primary immunodeficiencies treated with subcutaneous immunoglobulin (Pediatric Quality of Life Inventory [PedsQL], Child Health Questionnaire - Parent Form 50 [CHQ-PF50]11). We collected the data in a Microsoft Excel® spreadsheet. The aim of this phase is to assess differences in quality of life in relation to the use of each of these routes of administration.
Questionnaire developed specifically for assessing quality of life in patients with chronic hypogammaglobulinemia post allogeneic haematopoietic stem cell transplantation during past treatment with intravenous IgG and current treatment with subcutaneous IgG (minimum possible score of 0, corresponding to total satisfaction, and maximum of 52, corresponding to total dissatisfaction).
In the 2008–2019 period, a total of 175 HSCTs were performed in our hospital, with survival past 3 months post transplantation in 143 (82%). In the latter group, 3 patients (2%) exhibited persistent IgG deficiency more than 1 year after transplantation. All 3 required intravenous immunoglobulin replacement at the outset, which was eventually switched to the subcutaneous route.
The indication of HSCT was different in the 3 patients. Two required a second HSCT, one due to graft failure and the other due to haematologic relapse.
Table 1 summarises the variables analysed in the 3 patients.
Variables analysed in the 3 patients that exhibited persistent IgG deficiency more than 1 year after haematopoietic stem cell transplantation.
| Patient | Diagnosis | Chemotherapy regimens | C | GvHD | GvHD treatment | Rituximab |
|---|---|---|---|---|---|---|
| 1 | ALL | -SHOP/ALL 2015-ALL relapse SHOP/ALL 2008-Refractory ALLSEHOP 2008 | 1. MA: VP-16, CFM, TBI2. MA: TT, FLU, Bu | Grade III-IV(digestive tract, skin and liver) | CsA, MTX, steroids, MMF, B, ECP, TAC, SIR | Yes |
| 2 | Griscelli syndrome | HLH-2004: 3 cycles | 1. RIC: ALEM, FLU, MEL | Grade II (skin) | CsA, MMF, steroids | Yes |
| 3 | MPS type VII (Sly syndrome) | 1. RIC: ALEM, FLU, MEL2. MA: Bu, CFM, ATG | Grade II (skin) | CsA, MMF, steroids | Yes |
ALEM, alemtuzumab; ALL, acute lymphocytic leukaemia; ATG, antithymocyte globulin; B, budesonide; Bu, busulfan; CFM, cyclophosphamide; CsA, ciclosporin; ECP, extracorporeal photopheresis; FLU, fludarabine; GvHD, graft versus host disease; MA, myeloablative; MEL, melphalan; MMF, mycophenolate; MPS, mucopolysaccharidosis; MTX, methotrexate; RIC, reduced-intensity conditioning; SEHOP, Sociedad Española de Hematología y Oncología Pediátricas (Spanish Society of Paediatric Haematology and Oncology); SIR, sirolimus; TAC, tacrolimus; TBI, total body irradiation; TT, thiotepa; VP-16, etoposide.
Patient 1 (male): diagnosis of high-risk acute lymphocytic leukaemia type B at age 8 years. Management with chemotherapy with the SHOP/ALL 2005 protocol. Diagnosis of haematologic relapse at age 10 years, leading to implementation of chemotherapy protocol for relapsing disease (SHOP/ALL 2008) and the first bone marrow HSCT from an HLA-matched sibling donor (myeloablative conditioning with etoposide, cyclophosphamide and total body irradiation), GvHD prophylaxis with ciclosporin and methotrexate and prophylaxis against Pneumocystis jirovecii with cotrimoxazole. A second haematologic relapse was diagnosed at age 12 years, so the patient received chemotherapy following the SHOP/ALL 2008 protocol for refractory ALL (clofarabine, etoposide, cyclophosphamide, cytarabine and triple intrathecal therapy) and a second peripheral blood HSCT from the same donor (myeloablative conditioning with thiotepa, fludarabine and dose-adjusted busulfan), GvHD prophylaxis with ciclosporin and Pneumocystis jirovecii prophylaxis with cotrimoxazole. The main complications were reactivation of Epstein-Barr virus in the second transplantation that required treatment with rituximab and acute GvHD grade III-IV (with digestive tract, skin and liver involvement) that required addition of immunosuppression with steroids, mycophenolate, oral budesonide, extracorporeal photopheresis followed by tacrolimus and sirolimus.
Before transplantation, the patient had an IgG level of 256 mg/dL (normal range, 570−1570 mg/dL). He received prophylaxis with intravenous immunoglobulin from the first transplantation at increasing doses that went from 0.27 to 0.66 g/kg every 4 weeks (Privigen®), maintaining a mean IgG level during treatment of 677 mg/dL, with a trough at 190 mg/dL that coincided with a temporary suspension of treatment.
Immunoglobulin replacement therapy was switched to the subcutaneous route starting at a dose of 0.15 g/kg every 2 weeks (Hizentra®) 5 years after the second HSCT, with which he maintained mean IgG levels of 868 mg/dL with a trough of 447 mg/dL at 3 years of treatment, which led to an increase in the dose, with no further troughs detected. At the end of the study period, the patient was receiving a dose of 0.20 g/kg every 2 weeks.
Bacterial infections were not detected during intravenous or subcutaneous immunoglobulin replacement therapy.
As regards helper T cells, the patient had counts of 100–501 cells/μL in the first year post transplantation and 109–344 cells/μL in the 3 months following the second HSCT, after which the patient exhibited recovery with normal counts for age (10th percentile [P10], 640 helper T cells/μL).
The patient remains on complete remission after the second HSCT.
Patient 2 (male): diagnosis at age 4 years of Griscelli syndrome when the patient presented with haemophagocytic syndrome associated with Epstein-Barr infection, managed with the HLH-2004 protocol (dexamethasone, ciclosporin and etoposide). The patient experienced 3 relapses treated with the same protocol. At age 7 years, the patient underwent HSCT of peripheral blood of an unrelated donor with a 9/10 HLA match (difference in 1 locus B antigen) with reduced-intensity conditioning (alemtuzumab, fludarabine and melphalan), prophylaxis for GvHD with ciclosporin and mycophenolate, and prophylaxis for Pneumocystis jirovecii with cotrimoxazole. The main complications were acute GvHD grade II (skin involvement), treated with intravenous steroid therapy, and Epstein-Barr virus reactivation, treated with rituximab.
Before transplantation, the patient had an IgG level of 550 mg/dL (normal range, 571−1700 mg/dL). He received prophylaxis with intravenous immunoglobulin from the time of transplantation at increasing doses that ranged from 0.28 to 0.43/kg every 4 weeks (Privigen®), which achieved a sustained mean level of 676 mg/dL with the trough at 171 mg/dL, coinciding with the temporary suspension of treatment.
Immunoglobulin replacement therapy was switched to the subcutaneous route starting at a dose of 0.15 g/kg per week (Hizentra®) at 6 years post transplantation, which achieved mean IgG levels of 1094 mg/dL with no noteworthy troughs. The final dose during the study follow-up was of 0.20 g/kg per week.
Bacterial infections were not detected during treatment with intravenous or subcutaneous immunoglobulin.
As regards helper T cells, the patient had counts of 136–468 cells/μL for 7 months after transplantation, with subsequent recovery of normal counts for age (P10, 540 cells/μL).
The patient remains on complete remission after transplantation.
Patient 3 (female): diagnosis at age 1 year of mucopolysaccharidosis type VII (Sly syndrome). At age 2 years, she underwent an HLA-matched (10/10) unrelated donor bone marrow HSCT following reduced intensity conditioning (alemtuzumab, fludarabine and melphalan) and prophylaxis with ciclosporin for GvHD and with cotrimoxazole for Pneumocystis jirovecii. The main complications were reactivation of Epstein-Barr virus, treated with rituximab, and acute GvHD grade II (skin), which required intravenous steroid therapy and oral mycophenolate. Beta-glucuronidase levels normalised temporarily.
At age 3 years, there was evidence of graft failure, leading to a second HSCT of HLA-matched (10/10) unrelated donor umbilical cord blood following myeloablative conditioning (dose-adjusted busulfan, cyclophosphamide and ATG), GvHD prophylaxis with ciclosporin and Pneumocystis jirovecii prophylaxis with cotrimoxazole. The main complications were reactivation of Epstein-Barr virus, treated with rituximab on 2 occasions, and acute GvHD grade II-III (with involvement of skin and the digestive tract) that required treatment with steroids, oral budesonide and tacrolimus.
Before the first transplantation, the patient had an IgG level of 958 mg/dL (normal range, 383−1070 mg/dL). She received prophylaxis with intravenous immunoglobulin from the first transplantation at doses that increased mildly and then tapered over time, ranging from 0.37 g/kg every 2 weeks to 0.15 g/kg per week (Privigen®). She maintained a mean IgG level of 707 mg/dL, with a trough at 138 mg/dL that coincided with a temporary suspension of treatment.
Immunoglobulin replacement therapy was switched to the subcutaneous route starting at a dose of 0.17 g/kg every 2 weeks (Hizentra®) 4 years after the second transplantation, which achieved mean IgG levels of 623 mg/dL with no noteworthy troughs. The dose at the end of the study was 0.25 g/kg every 2 weeks.
Bacterial infections were not detected during treatment with intravenous or subcutaneous immunoglobulin.
As regards helper T cells, the patient had counts of 9.8–165 cells/μL for 6 months after the initial transplantation and 129–303 cells/μL for 10 months after the second transplantation, after which she exhibited normal counts for age (P10, 880 helper T cells/μL).
The patient has exhibited sustained complete donor chimerism with maintenance of normal levels of beta-glucuronidase from the second transplantation to present.
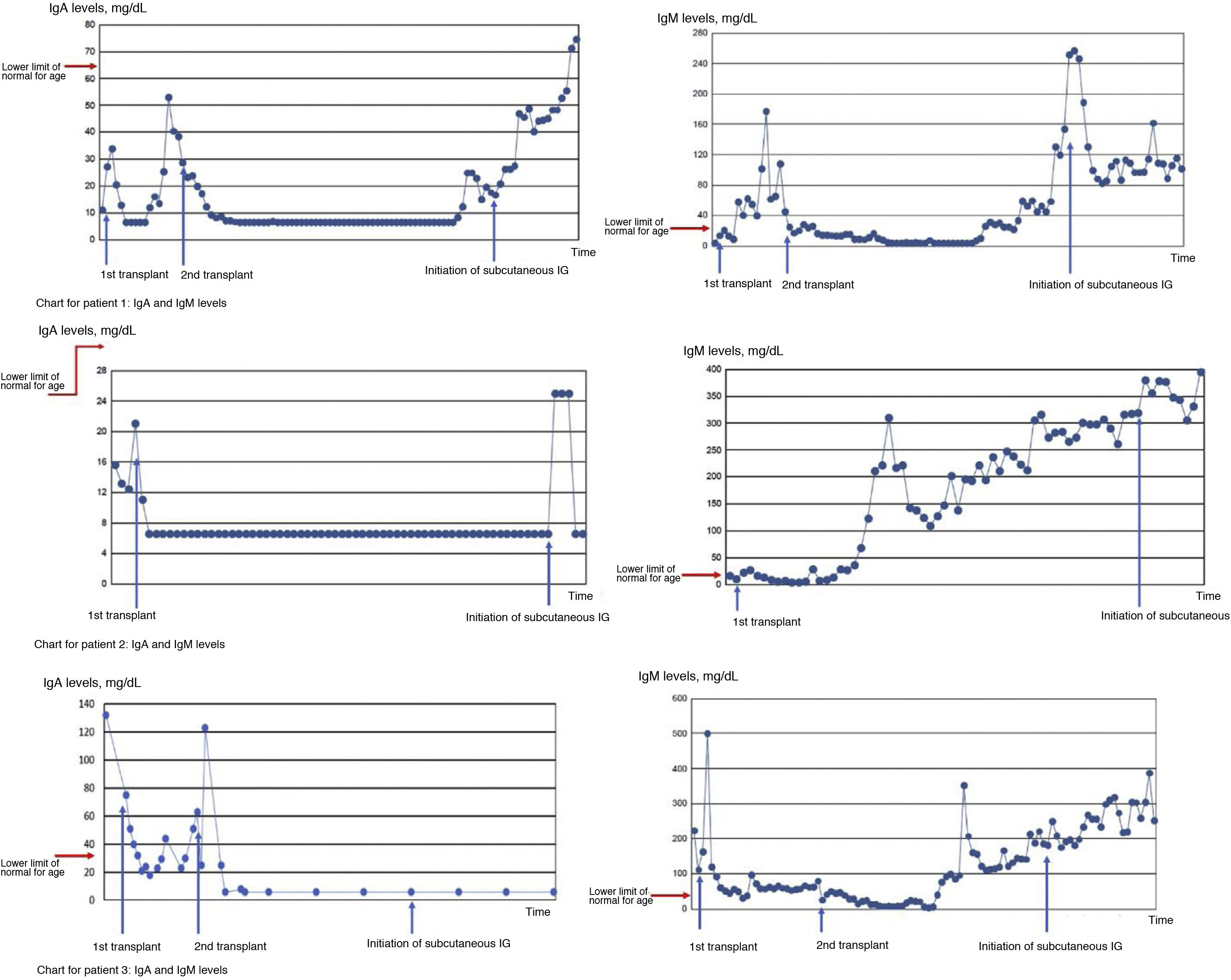
Fig. 2 presents charts showing the changes in IgA and IgM levels in the 3 patients during the follow-up. As can be seen in the chart for patient 1, IgA levels increased progressively from 4 years after the second transplantation, while IgM levels recovered in the period that elapsed between the transplantations and did not increase again until 4 years after the second one. In patient 2, IgA levels did not recover at any point to reach values in the normal range for age, while IgM levels did normalise for age from 1 year post HSCT. As for patient 3, the chart shows that IgA levels did not recover after the second transplantation, while IgM levels did recover from 14 months after the second transplantation.
Temporal trends in IgA and IgM in the 3 patients with chronic hypogammaglobulinemia after HSCT. In patient 1, IgA levels increased progressively starting from 4 years after the second transplantation, while IgM levels recovered in the period between transplantations and did not increase again until 4 years after the second transplantation. In patient 2, levels of IgA never recovered to reach the normal range for age, while levels of IgM reached the normal range for age from year 1 post HSCT. In patient 3, we found that IgA levels did not recover after the second transplantation, while IgM levels did recover from 14 months after the second transplantation.
Given the persistent need of intravenous immunoglobulin in each of these patients, B cell phenotype profiling in samples of peripheral blood was performed in all of them, revealing a considerable decrease in switched memory B cells, which are the producers of IgG and IgA, which could explain the chronic hypogammaglobulinaemia after transplantation. On the other hand, the percentage of naïve B cells was elevated. Table 2 presents the most recent results for each patient.
Peripheral blood B cell phenotype profiling in the 3 patients with chronic hypogammaglobulinemia after haematopoietic stem cell transplantation.
| B cells (CD19+)/µL | Switched memory B cells (IgD− CD27+) | Naïve (IgD+ CD27−) | |
|---|---|---|---|
| Patient 18 years after second transplant | 718 | 2.48%Normal range: P10 4%, P50 10%, P90 16% | 93.66% |
| Patient 25 years after transplant | 519 | 3%Normal range: P10 6%, P50 9%, P90 16% | 94% |
| Patient 37 years after second transplant | 477 | 0.43%Normal range: P10 6%, P50 9%, P90 16% | 97.22% |
Since patients exhibited chronic hypogammaglobulinaemia, treatment was switched to subcutaneous immunoglobulin (Hizentra®) for all 3 in year 2015. After educating the family and the patient on the correct technique to administer the immunoglobulin, treatment continued at home, with adequate tolerance and levels of IgG above 400 mg/dL throughout the follow-up in all 3 patients. No bacterial infections were detected during treatment.
In the quality of life survey conducted in the 3 patients and their parents, they all agreed in reporting satisfaction with subcutaneous delivery compared to intravenous delivery.
The mean score in the parental survey (intravenous immunoglobulin phase) was of 37 points, while the mean score in the patient survey (subcutaneous immunoglobulin phase) was of 2.6 points. In all items of the questionnaire, a higher score (maximum possible score: 52 points) indicates greater impairment (interference with everyday life activities, negative affect or sleep disturbances, interpersonal interactions and academic performance), while the lowest score (minimum possible, 0 points) indicates total satisfaction. All items received more positive ratings in the questionnaire completed by the patients themselves while receiving subcutaneous immunoglobulin, which evinces greater satisfaction with this route of delivery.
Patients valued not having to travel to hospital for the administration of treatment and being able to continue with their normal lives. We ought to highlight the comments added by patients 1 and 3, who also reported a substantial difference in the side effects experienced with intravenous immunoglobulin (headache and generalised pain in both cases) that did not occur with subcutaneous administration. None experienced local complications of subcutaneous immunoglobulin administration during the follow-up save for mild pain with needle insertion in the case of patient 3, who was the youngest.
DiscussionIn HSCT, immune reconstitution is associated with a hypogammaglobulinemia phase that tends to resolve in a few months, but in some cases this recovery does not occur, with persistence of chronic hypogammaglobulinemia beyond 1 year after HSCT. A study by Sudin et al.1,2 found proportions of 37%1 to 48%2 for hypogammaglobulinemia lasting more than 3 months after HSCT in children, although they did not report the incidence of persistent hypogammaglobulinemia of the form observed in our patients that required sustained replacement therapy found efficacious in preventing infection1. Although replacement therapy offers potential benefits, it requires numerous visits to the hospital of considerable duration, and has side effects and a high cost.
The aim of our study was to describe the characteristics of patients who develop chronic hypogammaglobulinaemia and to assess their satisfaction with the switch to subcutaneous immunoglobulin for chronic treatment. As a secondary objective, we aimed to assess the infections that developed during immunoglobulin replacement therapy and whether there were differences between the 2 routes of administration.
In our study of 175 patients, 3 (2%) developed post-transplantation chronic hypogammaglobulinaemia. These 3 patients shared factors described in the literature in relation to poor or incomplete immune reconstitution, such as young age at transplantation, underlying oncological or haematological disease, treatment with rituximab for Epstein-Barr virus reactivation, myeloablative conditioning (in 2 patients) and immunosuppressive therapy for GvHD.
Rituximab is a monoclonal antibody that binds the CD20 receptor in B cells. Naïve B cells (CD20+ IgD+ CD27−) develop in the bone marrow and migrate to secondary lymphoid organs to differentiate into switched memory B cells (CD20+ IgD− CD27+). Later on, they recirculate to the peripheral blood and toward lymphoid tissues or become plasma cells that stay in the bone marrow. The CD20 receptor is only expressed in B cells located in the peripheral blood and is not expressed in plasma cells. For this reason, antibodies against CD20 do not affect plasma cells and therefore do not result in complete depletion of B cells12.
Low counts of switched memory B cells and an increase in naïve B cells, as seen in our patients, have been described during reconstitution after treatment with rituximab12. This suggests that the predominance of naïve B cells during reconstitution could contribute to the duration of hypogammaglobulinemia. This population includes regulatory B cells that prevent haematologic relapse by promoting immune tolerance through the production of interleukin (IL) 10, IL35 and tumour growth factor beta (TGF-β), which inhibit pro-inflammatory lymphocyte expansion12. In the most recent evaluations, our patients continued to have abnormally high counts of naïve B cells and switched memory B cell counts below the tenth percentile for age13, which explained the persistence of low levels of IgG and the need for immunoglobulin replacement.
The reported incidence of transient or persistent hypogammaglobulinemia after the use of rituximab in adults ranges from 15% to 40%3. Other concomitant factors have been described in patients with persistent hypogammaglobulinaemia, such as regimens that included fludarabine, cyclophosphamide or mycophenolate mofetil (the latter inhibits nucleic acid synthesis and prevents activation of B and T cells)14. These 3 drugs were used in the management of our patients. In other cases, hypogammaglobulinaemia results from a masked primary immunodeficiency12, so the aetiology of persistent hypogammaglobulinaemia is considered to be multifactorial.
In the study by Sudin et al.2, persistent hypogammaglobulinaemia was more frequent in younger patients, patients with GvHD and patients with higher pre-transplantation levels of IL-6 and IL-7. These interleukins keep B cells in their most immature stage or in transitional stages and prevent immunoglobulin class switching, which would explain why patients with low levels of IgG have adequate levels of IgM and low levels of IgA, as was also the case in our patients. Another aspect to be taken into account is that low counts of helper T cells after HSCT, as seen in our patients, contribute to hypogammaglobulinemia G. Helper T cells are necessary for isotype switching, and in their absence, B cells are activated by thymus-independent antigens, thus producing IgM but not IgA or IgG.
After HSCT, patients are more vulnerable to viral, bacterial and fungal infections. This increased risk is partly due to the hypogammaglobulinemia that develops in the initial period following HSCT. Usually, B cell recovery occurs between 6 and 9 months and IgM recovery between 4 and 6 months post transplantation, while normalization of IgG may take up to 12 months or longer if complications develop1, so that prophylactic intravenous IgG replacement is necessary in these cases. Although current guidelines do not recommend routine administration of immunoglobulin to all patients, they do recommend maintenance of IgG levels at or above 400 mg/dL to reduce the incidence of infection, which has proven effective in numerous studies1,15.
In our sample, the 3 patients received intravenous IgG for prophylaxis and maintained levels greater than 400 mg/dL while hypogammaglobulinaemia persisted. After switching to the subcutaneous route, there were no differences in the incidence of bacterial infection, as the patients remained free of this complication throughout the follow-up.
Many studies have assessed the efficacy of subcutaneous immunoglobulin in adults and children under 12 years10,16 with primary and secondary immunodeficiencies10,17–22, or even in children aged less than 5 years20, and have not found statistically significant differences in the frequency or severity of infections18,21,22.
Sudin et al.1 compared the use of subcutaneous and intravenous immunoglobulin in paediatric patients that underwent HSCT, and found that patients that received subcutaneous immunoglobulin had more stable IgG levels, experienced fewer adverse events and did not have a significantly different number of infections. At the family level, the subcutaneous treatment and the associated reduction in the frequency of medical visits were perceived positively. Families did not experience difficulties in learning how to administer immunoglobulin subcutaneously.
Within the constraints imposed by the limitations of our study (small sample size, use of a questionnaire that has not been validated, with retrospective data collection, and administered to parents in the first phase and to the patients in the second), we found that in all 3 patients, the switch from intravenous to subcutaneous immunoglobulin substantially improved quality of life. Although the questionnaire we used has not been validated, our results are consistent with the previous literature, with an increase in reported patient quality of life and satisfaction23–25 in both adult and paediatric patients with a primary immunodeficiency treated with subcutaneous immunoglobulin.
In addition to the demonstrated efficacy of subcutaneous immunoglobulin and the positive perception of the treatment, there is also evidence of a reduction in costs26–29 and in the number of necessary hospital visits. Thus, it seems reasonable to offer this treatment to patients that do no longer carry a central venous catheter and continue to require immunoglobulin replacement therapy after transplantation but do not need to be hospitalised.
ConclusionDespite the limited number of patients in our study, which precludes drawing conclusions at the population level, we can assert that subcutaneous immunoglobulin replacement therapy with Hizentra® at 20%, was as effective as intravenous immunoglobulin therapy without evidence of an increase in bacterial infection. It was also associated with an improvement in quality of life and increased comfort, as reported by patients. The flexibility afforded by this approach constitutes a significant advance for paediatric patients whose follow-up requires regular visits to the hospital.
As regards the aetiology of chronic hypogammaglobulinemia after HSCT, we identified different factors in our patients that could explain its development. We ought to highlight the use of rituximab as the most evidently and directly associated with switched memory B cell recovery. Still, the 3 patients shared several other concomitant factors and therefore it is not possible to attribute the development of chronic hypogammaglobulinaemia to that sole factor.
FundingIgMAPs grant awarded on 13/09/17.
Conflicts of interestThe study was conducted thanks to the support of a IgMAPs grant from the CSL Behring laboratory; CSL Behring provided medical writing support and funds, and played no role in the review or the development of the manuscript.
Please cite this article as: Serra Font S, López-Granados L, Sisinni L, Serna Berna JV, Martínez Martínez L, Fernández de Gamarra-Martínez E, et al. Hipogammaglobulinemia crónica post trasplante de precursores hematopoyéticos y su tratamiento con gammaglobulina subcutánea en pacientes pediátricos. An Pediatr (Barc). 2022;97:103–111.