To assess the frequency of the multiple organ failure and the prognostic value of multiple organ failure scores in children who have recovered from an in-hospital cardiac arrest.
Patients and methodsA single centre, observational, and retrospective study was conducted on children between 1 month and 16 years old who suffered an in-hospital cardiac arrest and achieved return of spontaneous circulation (ROSC). In the first 24–48h and between the fifth and the seventh day after ROSC, a record was made of the scores on paediatric severity (PRISM and PIM II) and multiple organ failure scales (PELOD and P-MODS), along with the clinical and analytical data, and including monitoring and treatment, mortality and cause of death.
ResultsOf the total of 41 children studied, 70.7% were male, and the median age was 38 months. The overall mortality during admission was 41.5%, with 14.6% dying in the first 48h, and 7.6% in the following 3–5 days. In the first 48h, clinical severity and multiple organ failure scores were higher in the patients that died than in survivors (PRISM 29 vs. 21) p=0.125, PIM II (26.8% vs. 9.2%) p=0.02, PELOD (21 vs. 12) p=0.005, and P-MODS (9 vs. 6) p=0.001. Between the fifth and seventh day, the scores on the four scales were also higher in patients who died, but only those of the PELOD (20.5 vs. 11) p=0.002 and P-MODS (6.5 vs. 3) p=0.003 reached statistical significance.
ConclusionsMortality in children after return of spontaneous circulation after cardiac arrest is high. The multiple organ failure after return of spontaneous circulation after cardiac arrest in children is associated with increased mortality.
Estudiar la incidencia del fallo multiorgánico (FMO) y el valor pronóstico de las puntuaciones de FMO en los niños que se han recuperado de una parada cardiaca (PC) intrahospitalaria.
Pacientes y métodosEstudio unicéntrico, observacional, retrospectivo, en niños menores de 16 años, que presentaron una PC intrahospitalaria y alcanzaron la recuperación de la circulación espontánea (RCE). Se registraron las puntuaciones de las escalas de gravedad (PRISM y PIM II) y FMO (PELOD y P-MODS), la mortalidad y la causa del fallecimiento.
ResultadosSe estudió a 41 niños (70,7% varones), con una mediana de edad de 38 meses. Durante el ingreso falleció el 41,5% (el 14,6% en las primeras 48h y un 7,3% en los siguientes 5 días). En las primeras 48h, las puntuaciones de gravedad clínica y de FMO fueron más altas en los fallecidos que en los supervivientes (PRISM 29 frente a 21), p=0,125, PIM II (26,8% frente a 9,2%), p=0,021, PELOD (21 frente a 12), p=0,005, y P-MODS (9 frente a 6), p=0,001. Entre el 5.° y el 7.° día las puntuaciones de las 4 escalas fueron también mayores en los fallecidos, pero solo las escalas PELOD (20,5 frente a 11), p=0,002, y P-MODS (6,5 frente a 3), p=0,003, alcanzaron significación estadística.
ConclusionesLa mortalidad de los niños que se recuperan de una PC es elevada. El FMO tras la RCE de una PC en el niño se asocia a una mayor mortalidad.
Cardiac arrest (CA) is defined as a sudden, unexpected and potentially reversible stop of spontaneous blood flow and respiration.1 The incidence of in-hospital CA in children ranges between 0.19 and 2.45 per 1000 hospital admissions.2
The goal of cardiopulmonary resuscitation (CPR) is the return of spontaneous circulation (ROSC). In adults, more than half of the patients that achieve ROSC do not survive to discharge, and the most frequent causes of death are brain death and multiple organ failure (MOF).3
Multiple organ failure is defined as failure of two or more organ systems that cannot sustain their activity spontaneously. It is the leading cause of death in intensive care units both in children and adults.4
Following CA, all organs are affected by an ischaemia–reperfusion phenomenon that increases the risk of MOF. Several studies in adults have reported that the development of acute kidney failure,5 acute adrenal insufficiency6,7 or disseminated intravascular coagulation, higher scores in disease severity scales such as APACHE II8,9 or MOF are associated with increased mortality.10 However, only one study in adults has described a high incidence of MOF after CA associated with an increased mortality that appeared to be driven by haemodynamic dysfunction and oxygenation impairment.3
There are no studies in children analysing the prevalence of MOF after CA or whether the development of MOF post CA carries a poorer prognosis.
The aim of our study was to analyse the outcomes of children that achieve ROSC after CA, determine the incidence of MOF, and assess the predictive power of the Pediatric Risk of Mortality (PRISM)11 and Pediatric Index of Mortality II (PIM II) disease severity scores, the Pediatric Multi Organ Dysfunction Score (P-MODS)12 and Pediatric Logistic Organ Dysfunction (PELOD)13 paediatric MOF scores and other monitoring and laboratory parameters that may be altered following ROSC,14–24 as well as the causes of death.25
Patients and methodsWe conducted a single-centre observational retrospective study in which we included children that experienced in-hospital CA and achieved ROSC between December 2007 and June 2013.
The inclusion criteria were: age 1 month to 16 years, in-hospital CA with ROSC after CPR. We excluded children that had out-of-hospital CA and children that did not achieve ROSC after CPR.
We collected data for the following variables: age, sex, weight, history of preterm birth, heart, respiratory, neurologic, gastrointestinal, or renal disease, cancer, blood disorder, congenital anomalies or surgery. We documented the past history of CA, whether the patient had required treatment with angiotensin-converting enzyme (ACE) inhibitors in the past, the history of organ failure prior to CA, the cause and type of CA (cardiac or respiratory), ECG rhythm, setting of diagnosis (paediatric ICU, inpatient ward, operating room, emergency room or other), time elapsed between arrest and initiation of CPR, and duration of CPR.
The following data were collected in the first 24–48h and between day five and seven post CA: predominant ECG rhythm, echocardiography (if performed), blood pressure (BP), heart rate (HR), breathing rate, body temperature, diuresis, administration and dosage of inotropes, sedatives, insulin and antibiotics, type of nutrition, mechanical ventilation, peak flow rate, FiO2 and end expiratory pressure; continuous renal replacement therapy (CRRT), ventricular assist devices and extracorporeal membrane oxygenation (ECMO), presence of infection and its location, haemorrhage and/or pneumothorax.
We also collected data for the following laboratory variables: arterial and/or venous blood gases (PO2, PCO2, O2 saturation, pH, bicarbonate and base excess), chemistry panel (glucose, ions, transaminases, total bilirubin, bicarbonate, lactate, creatinine, urea, C-reactive protein, procalcitonin, BNP, troponin, CPK-MB, cortisol) blood counts (haemoglobin, white blood cells, platelets) and coagulation tests (INR, aPTT, fibrinogen, D dimer). To assess neurologic outcomes, we collected data for seizures, evaluation of pupillary response, continuous EEG monitoring, bispectral index (BIS), cerebral oxygen monitoring, findings of computed tomography or magnetic resonance imaging, and use of therapeutic hypothermia including time elapsed between CA and initiation of hypothermia and its duration. For patients that died, we collected the cause of death and the days elapsed between CA and death.
We calculated the prognostic scores at 24–48h and at day five to seven after ROSC, using the PRISM,11 PELOD,13 P-MODS12 and PIM II paediatric scales.
We conducted the statistical analysis with the software SPSS, version 21. We have expressed the data using the median and interquartile range (IQR) as they did not follow a normal distribution. We analysed the association between PRISM, PELOD, P-MODS and PIM II scores and mortality by comparing the number of deceased and surviving patients with the nonparametric Mann–Whitney U test. Statistical significance was defined as a p-value of less than 0.05. We analysed survival by means of Kaplan–Meier curves. We performed multivariate survival analysis by means of Cox regression, excluding the confounding variables of the selected reduced model.
ResultsWe analysed data for 41 patients with a median age of 38 months (IQR, 5.5–94 months) and a median weight of 12kg (IQR, 5–22kg). Seventy percent were male.
Past historyNinety percent of the children had a past history of disease (heart disease in 75%, preterm birth in 15%, respiratory disease in 7.5%, neurologic in 5%, gastrointestinal in 10%, renal in 5%, and cancer in 2.5%; 17.1% had congenital anomalies, 10% had a history of recurrent infection, 46.3% had multiple prior surgeries and 9.6% presented with malnutrition). We found a prior history of CA in 17.5% of patients, and 27.5% were being treated with ACE inhibitors. Of all patients, 47.5% had a history of MOF that predated CA.
Characteristics of cardiac arrest and cardiopulmonary resuscitationThe aetiology of CA was cardiovascular in 62.5% of patients, respiratory in 27.5%, infectious in 5%, neurologic in 2.5% and other in the remaining 2.5%. In 74.4%, the arrest originated in the heart and in 25.6% in the respiratory system.
The setting where CA took place was the paediatric ICU in 69.2% of cases, the operating room in 12.8%, the emergency department in 2.6% and elsewhere in the hospital in 15.4%.
In 97.4% of the patients, CPR was initiated within 1min of CA, and in 2.6% between 4 and 10min post CA.
The cardiac rhythm at the time of CA was asystole or severe bradycardia in 65% of patients, pulseless electrical activity in 22.5% and ventricular fibrillation or pulseless ventricular tachycardia in 12.5%. In 69.7% of patients, the duration of the arrest was less than 10min, with a median duration of four minutes (IQR, 1.5–15min).
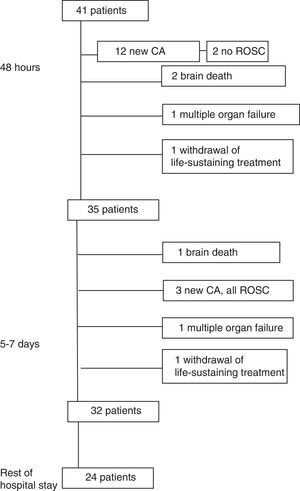
Patient outcomes after return of spontaneous circulationSeventeen children (41.5%) died during their stay. The time elapsed between ROSC and death was 9.5 days (IQR, 2–19.7 days).
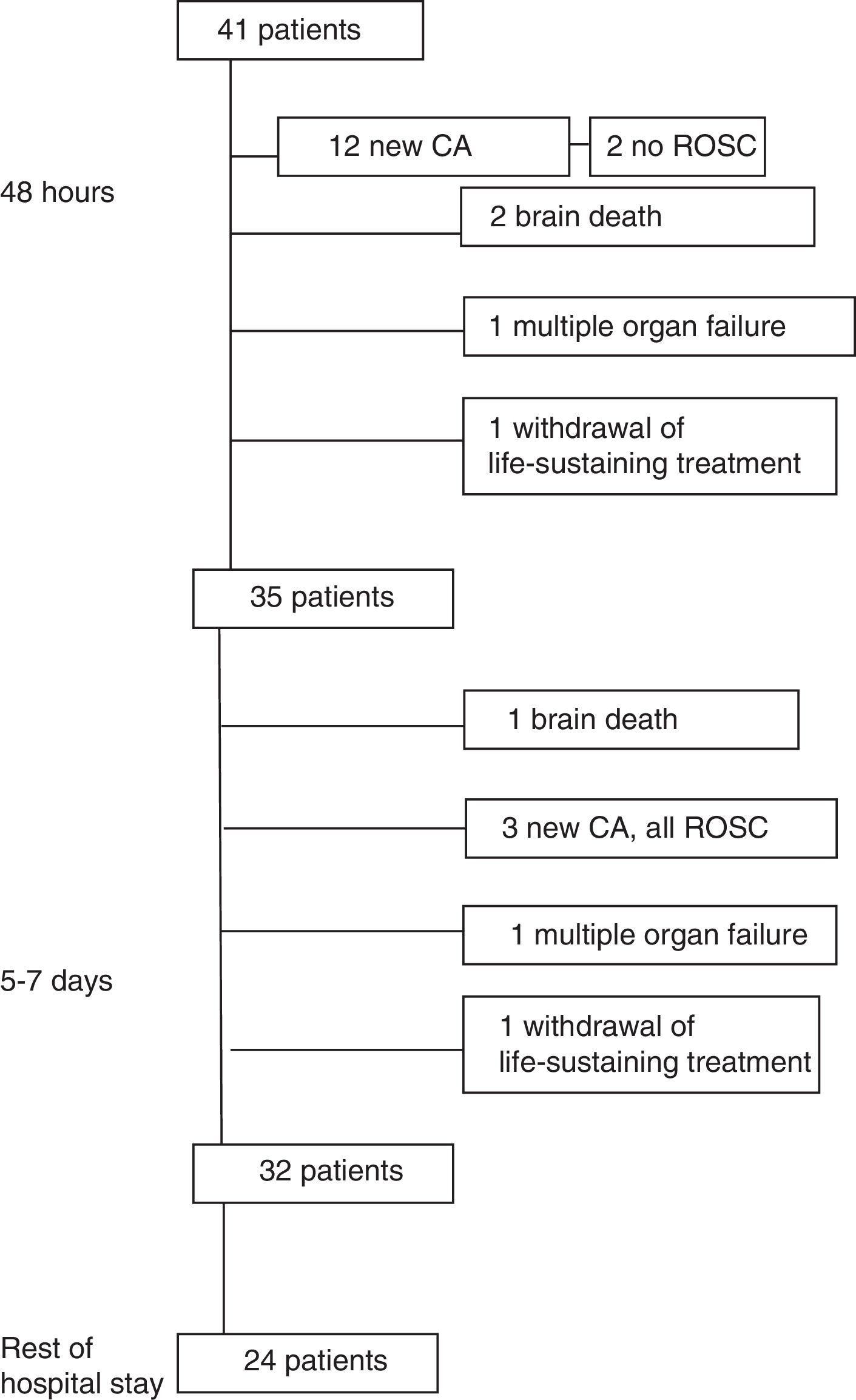
Outcomes in the first 24–48hFig. 1 summarises patient outcomes.
In the first 24–48h after ROSC, 12 patients (29.3%) had another CA. Six patients (14.6%) died in the first 24–48h following ROSC (two due to a new CA without ROSC, two due to brain death, one due to MOF and one following withdrawal of life-sustaining treatment).
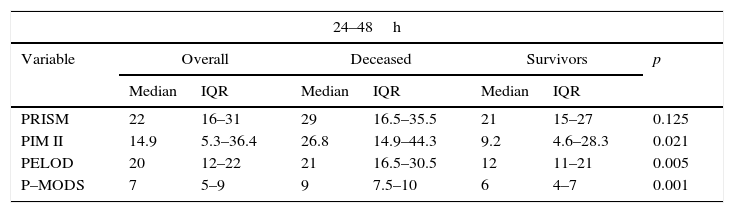
The PIM II, PELOD and P-MODS scores of children that died were significantly higher than those of survivors. The PRISM score was also higher in children that died compared to survivors, but the difference was not statistically significant (Table 1).
Comparison of disease severity and multiple organ failure scores in patients that died vs. survivors.
| 24–48h | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Overall | Deceased | Survivors | p | |||
| Median | IQR | Median | IQR | Median | IQR | ||
| PRISM | 22 | 16–31 | 29 | 16.5–35.5 | 21 | 15–27 | 0.125 |
| PIM II | 14.9 | 5.3–36.4 | 26.8 | 14.9–44.3 | 9.2 | 4.6–28.3 | 0.021 |
| PELOD | 20 | 12–22 | 21 | 16.5–30.5 | 12 | 11–21 | 0.005 |
| P–MODS | 7 | 5–9 | 9 | 7.5–10 | 6 | 4–7 | 0.001 |
| 5–7 days | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Overall | Deceased | Survivors | p | |||
| Median | IQR | Median | IQR | Median | IQR | ||
| PRISM | 14 | 8.5–18.5 | 17 | 13.5–20.2 | 11 | 8–18 | 0.114 |
| PIM II | 6.9 | 2.7–19.4 | 14 | 4.8–31.6 | 6.5 | 2.1–16.9 | 0.103 |
| PELOD | 11 | 11–20 | 20.5 | 13.2–21.7 | 11 | 10–11 | 0.002 |
| P–MODS | 4 | 3–6 | 6.5 | 4.5–10 | 3 | 2–4.2 | 0.003 |
Of all patients, 97.6% were treated with sedation, inotropic therapy and mechanical ventilation. Bilaterally dilated pupils were observed in 5.4% of patients, and moderate to small pupillary reactions in 16.2%. Seizures were documented in 4.9% of patients.
The inotropic score was higher in patients that died (median, 50) compared to survivors (median, 41), although the difference was not statistically significant (p=0.2). We also found no differences in lactate clearance (0.85 in survivors vs. 0.75 in deceased patients; p=0.11).
Outcomes at five to seven daysBetween days five and seven after ROSC, three patients (7.3%) had a new CA, and all recovered.
Three patients (7.3% of the total) died between days five and seven (one due to MOF, one due to brain death, and one following withdrawal of life-sustaining treatment). Eight patients died after day seven (four due to MOF, 2 due to a new CA from which they did not recover, one from a brain haemorrhage and one due to pulmonary haemorrhage).
The PELOD and P-MODS scores of the children that died were significantly higher compared to those of survivors. The PRISM and PIM II scores were also higher in deceased patients, but the difference was not statistically significant (Table 1).
The inotropic score at five to seven days of patients that died was significantly higher (43.5; IQR, 40–65) compared to survivors (25; IQR, 18.2–30.2) (p=0.001). There was no difference in lactate clearance between survivors (0.36) and deceased patients (0.28) (p=0.122).
Pupillary reactions were moderate to small in 3.7% of patients, and normal in all others. At this time, 89.3% of patients required sedation, 92.9% inotropic therapy and 75% mechanical ventilation.
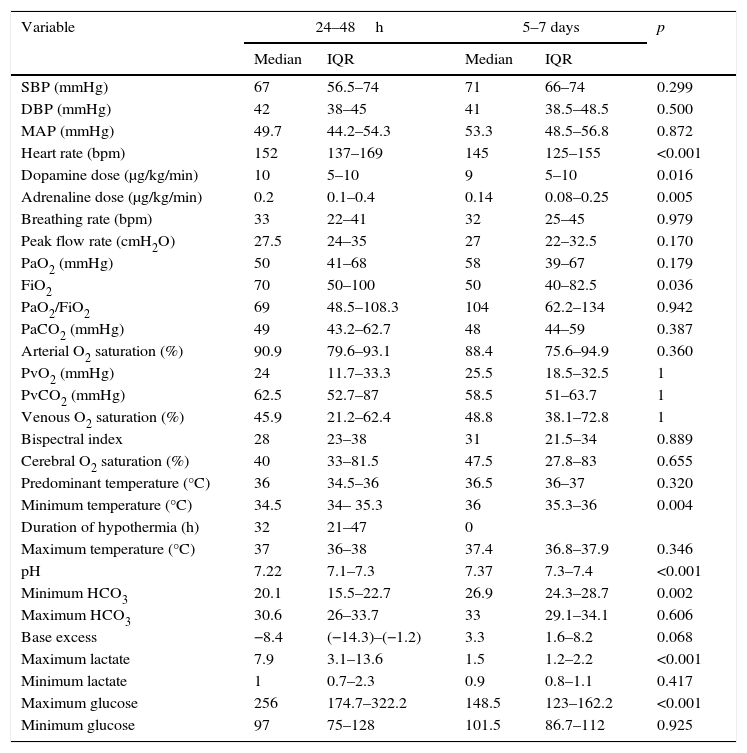
Comparison of variables over timeTable 1 summarises the scores in the PRISM and PIM II paediatric disease severity scales as well as the MOF PELOD and P-MODS. The scores in all scales fell between the two time points of 24–48h and days five to seven (PRISM, p<0.001; PIM II, p=0.011; PELOD, p=0.004; P-MODS, p=0.001).
Table 2 presents the median and IQR of the main variables under study at 24–48h and days five to seven from ROSC.
Changes in haemodynamic, respiratory, neurologic and laboratory parameters.
| Variable | 24–48h | 5–7 days | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| SBP (mmHg) | 67 | 56.5–74 | 71 | 66–74 | 0.299 |
| DBP (mmHg) | 42 | 38–45 | 41 | 38.5–48.5 | 0.500 |
| MAP (mmHg) | 49.7 | 44.2–54.3 | 53.3 | 48.5–56.8 | 0.872 |
| Heart rate (bpm) | 152 | 137–169 | 145 | 125–155 | <0.001 |
| Dopamine dose (μg/kg/min) | 10 | 5–10 | 9 | 5–10 | 0.016 |
| Adrenaline dose (μg/kg/min) | 0.2 | 0.1–0.4 | 0.14 | 0.08–0.25 | 0.005 |
| Breathing rate (bpm) | 33 | 22–41 | 32 | 25–45 | 0.979 |
| Peak flow rate (cmH2O) | 27.5 | 24–35 | 27 | 22–32.5 | 0.170 |
| PaO2 (mmHg) | 50 | 41–68 | 58 | 39–67 | 0.179 |
| FiO2 | 70 | 50–100 | 50 | 40–82.5 | 0.036 |
| PaO2/FiO2 | 69 | 48.5–108.3 | 104 | 62.2–134 | 0.942 |
| PaCO2 (mmHg) | 49 | 43.2–62.7 | 48 | 44–59 | 0.387 |
| Arterial O2 saturation (%) | 90.9 | 79.6–93.1 | 88.4 | 75.6–94.9 | 0.360 |
| PvO2 (mmHg) | 24 | 11.7–33.3 | 25.5 | 18.5–32.5 | 1 |
| PvCO2 (mmHg) | 62.5 | 52.7–87 | 58.5 | 51–63.7 | 1 |
| Venous O2 saturation (%) | 45.9 | 21.2–62.4 | 48.8 | 38.1–72.8 | 1 |
| Bispectral index | 28 | 23–38 | 31 | 21.5–34 | 0.889 |
| Cerebral O2 saturation (%) | 40 | 33–81.5 | 47.5 | 27.8–83 | 0.655 |
| Predominant temperature (°C) | 36 | 34.5–36 | 36.5 | 36–37 | 0.320 |
| Minimum temperature (°C) | 34.5 | 34– 35.3 | 36 | 35.3–36 | 0.004 |
| Duration of hypothermia (h) | 32 | 21–47 | 0 | ||
| Maximum temperature (°C) | 37 | 36–38 | 37.4 | 36.8–37.9 | 0.346 |
| pH | 7.22 | 7.1–7.3 | 7.37 | 7.3–7.4 | <0.001 |
| Minimum HCO3 | 20.1 | 15.5–22.7 | 26.9 | 24.3–28.7 | 0.002 |
| Maximum HCO3 | 30.6 | 26–33.7 | 33 | 29.1–34.1 | 0.606 |
| Base excess | −8.4 | (−14.3)–(−1.2) | 3.3 | 1.6–8.2 | 0.068 |
| Maximum lactate | 7.9 | 3.1–13.6 | 1.5 | 1.2–2.2 | <0.001 |
| Minimum lactate | 1 | 0.7–2.3 | 0.9 | 0.8–1.1 | 0.417 |
| Maximum glucose | 256 | 174.7–322.2 | 148.5 | 123–162.2 | <0.001 |
| Minimum glucose | 97 | 75–128 | 101.5 | 86.7–112 | 0.925 |
DBP, diastolic arterial blood pressure; FiO2, fraction of inspired oxygen; IQR, interquartile range; MAP, mean arterial pressure; PaCO2, arterial partial carbon dioxide pressure; PaO2, arterial partial oxygen pressure; PvCO2, venous partial carbon dioxide pressure; PvO2, venous partial oxygen pressure; SBP, arterial systolic blood pressure.
We found no significant differences in the haemodynamic parameters (BP and HR) or the dosage of inotropic agents between the two time points. The FiO2 was higher in the first 48h compared to days five to seven (p=0.036). There were no statistically significant differences in any of the remaining respiratory parameters. Therapeutic hypothermia was used in 19 patients in the first 48h. There were no significant differences in BIS and cerebral oxygen saturation values between the two periods. At the day five-to-seven time point, fibrinogen levels had increased (p=0.025) while the INR had decreased (p=0.037). Urine volume was greater in the first 48h (p=0.005). As for liver function parameters, AST levels were higher (p=0.035) and GGT levels lower (p=0.001) in the first 48h. Electrolyte levels, blood gases, pH (p=0.001) and bicarbonate levels (p=0.002) were lower in the 48h, while lactate and glucose levels were higher (p<0.001 for both).
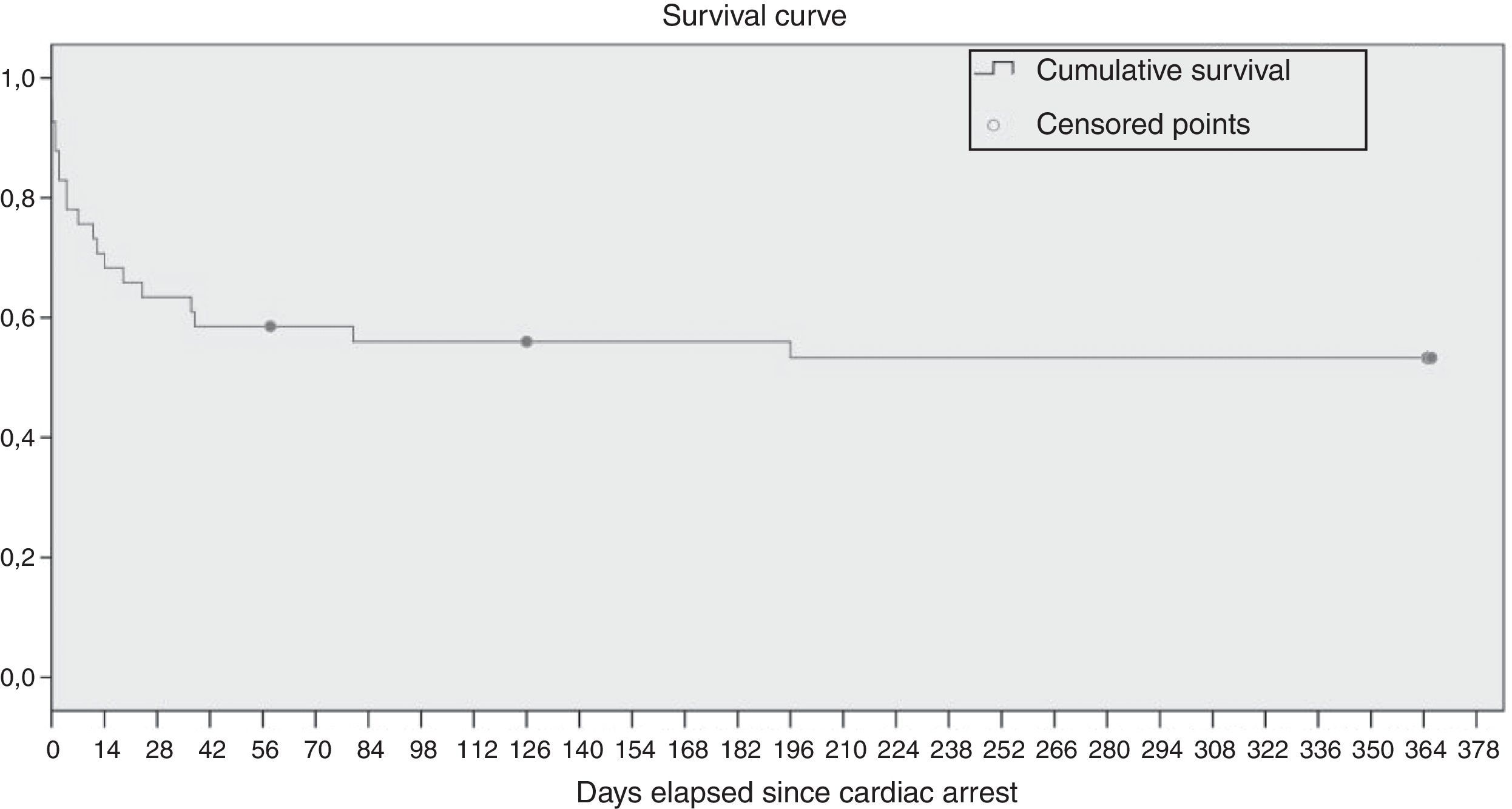
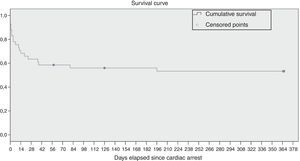
Survival analysisThe Kaplan–Meier survival analysis found a survival of 82.9% of the patients in the seven days following CA. The mean survival was 207 days (CI, 154–260). Fig. 2 shows the survival curve.
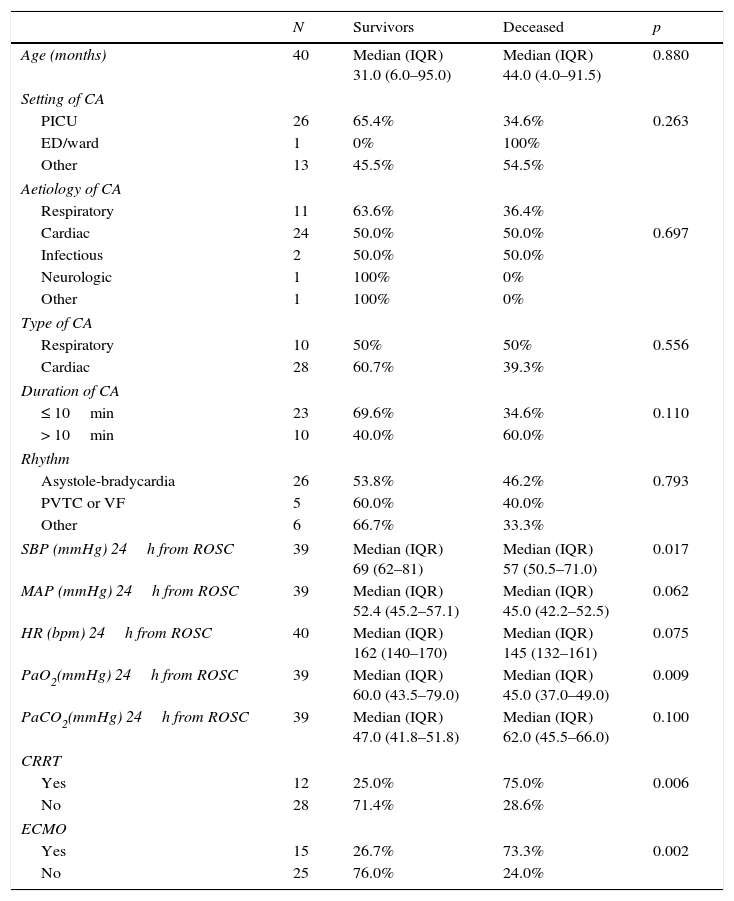
Table 3 summarises the main differences in the variables under study between deceased patients and survivors. In children that died, systolic BP and PaO2 at 24h were significantly lower, while the need for ECMO and CRRT were higher.
Most relevant differences between deceased patients and survivors.
| N | Survivors | Deceased | p | |
|---|---|---|---|---|
| Age (months) | 40 | Median (IQR) 31.0 (6.0–95.0) | Median (IQR) 44.0 (4.0–91.5) | 0.880 |
| Setting of CA | ||||
| PICU | 26 | 65.4% | 34.6% | 0.263 |
| ED/ward | 1 | 0% | 100% | |
| Other | 13 | 45.5% | 54.5% | |
| Aetiology of CA | ||||
| Respiratory | 11 | 63.6% | 36.4% | |
| Cardiac | 24 | 50.0% | 50.0% | 0.697 |
| Infectious | 2 | 50.0% | 50.0% | |
| Neurologic | 1 | 100% | 0% | |
| Other | 1 | 100% | 0% | |
| Type of CA | ||||
| Respiratory | 10 | 50% | 50% | 0.556 |
| Cardiac | 28 | 60.7% | 39.3% | |
| Duration of CA | ||||
| ≤ 10min | 23 | 69.6% | 34.6% | 0.110 |
| > 10min | 10 | 40.0% | 60.0% | |
| Rhythm | ||||
| Asystole-bradycardia | 26 | 53.8% | 46.2% | 0.793 |
| PVTC or VF | 5 | 60.0% | 40.0% | |
| Other | 6 | 66.7% | 33.3% | |
| SBP (mmHg) 24h from ROSC | 39 | Median (IQR) 69 (62–81) | Median (IQR) 57 (50.5–71.0) | 0.017 |
| MAP (mmHg) 24h from ROSC | 39 | Median (IQR) 52.4 (45.2–57.1) | Median (IQR) 45.0 (42.2–52.5) | 0.062 |
| HR (bpm) 24h from ROSC | 40 | Median (IQR) 162 (140–170) | Median (IQR) 145 (132–161) | 0.075 |
| PaO2(mmHg) 24h from ROSC | 39 | Median (IQR) 60.0 (43.5–79.0) | Median (IQR) 45.0 (37.0–49.0) | 0.009 |
| PaCO2(mmHg) 24h from ROSC | 39 | Median (IQR) 47.0 (41.8–51.8) | Median (IQR) 62.0 (45.5–66.0) | 0.100 |
| CRRT | ||||
| Yes | 12 | 25.0% | 75.0% | 0.006 |
| No | 28 | 71.4% | 28.6% | |
| ECMO | ||||
| Yes | 15 | 26.7% | 73.3% | 0.002 |
| No | 25 | 76.0% | 24.0% | |
CA, cardiac arrest; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ED, emergency department; HR, heart rate; IQR, interquartile range; MAP, mean arterial pressure; PICU, paediatric intensive care unit; PVTC, pulseless ventricular tachycardia; ROSC, return of spontaneous circulation; SBP, systolic blood pressure; VF, ventricular fibrillation.
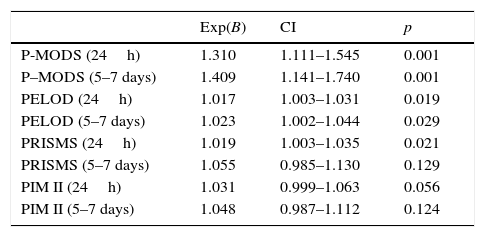
Table 4 shows the association of disease severity and MOF risk scales with survival. The P-MODS was the scale with the strongest correlation with survival. In the first 24h, each one-point increase in this score was associated with a 30.9% increase in mortality risk (p=0.001) compared to an increase of 2% per point in the PELOD (p=0.019) or the PRISM (p=0.021). The analysis of the PIM II scores did not find statistically significant differences.
Survival analysis.
| Exp(B) | CI | p | |
|---|---|---|---|
| P-MODS (24h) | 1.310 | 1.111–1.545 | 0.001 |
| P–MODS (5–7 days) | 1.409 | 1.141–1.740 | 0.001 |
| PELOD (24h) | 1.017 | 1.003–1.031 | 0.019 |
| PELOD (5–7 days) | 1.023 | 1.002–1.044 | 0.029 |
| PRISMS (24h) | 1.019 | 1.003–1.035 | 0.021 |
| PRISMS (5–7 days) | 1.055 | 0.985–1.130 | 0.129 |
| PIM II (24h) | 1.031 | 0.999–1.063 | 0.056 |
| PIM II (5–7 days) | 1.048 | 0.987–1.112 | 0.124 |
CI, confidence interval.
Between days five and seven, mortality risk increased by 40.9% with each one-point increase in the P-MODS score (p=0.001) compared to 2.3% for the PELOD scale (p=0.029), while the association was not statistically significant for the PRISM and PIM II scores.
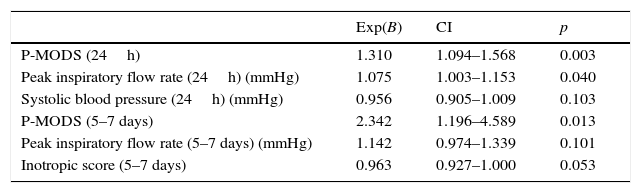
Table 5 presents the results of the multivariate analysis for both time periods.
Multivariate survival analysis.
| Exp(B) | CI | p | |
|---|---|---|---|
| P-MODS (24h) | 1.310 | 1.094–1.568 | 0.003 |
| Peak inspiratory flow rate (24h) (mmHg) | 1.075 | 1.003–1.153 | 0.040 |
| Systolic blood pressure (24h) (mmHg) | 0.956 | 0.905–1.009 | 0.103 |
| P-MODS (5–7 days) | 2.342 | 1.196–4.589 | 0.013 |
| Peak inspiratory flow rate (5–7 days) (mmHg) | 1.142 | 0.974–1.339 | 0.101 |
| Inotropic score (5–7 days) | 0.963 | 0.927–1.000 | 0.053 |
CI, confidence interval.
In the first 24h, the only variable associated with survival was the ventilator peak inspiratory flow rate, with the risk of mortality increasing by 7.5% with each unit increase in pressure (CI, 1.003–1.153; p=0.04). We did not find a statistically significant association for any of the variables analysed for days five to seven.
DiscussionIn-hospital cardiopulmonary arrest in the paediatric age group carries a high mortality. The percentage of children that survive CA is highly variable.1,26–30 A considerable proportion do not recover from CA, while other children achieve ROSC but die within a few days or weeks.
The survival of children that achieved ROSC after CA in our study was 58.5%, a proportion that was similar to those reported in other recent studies in the paediatric pouplation.26–30
This is the first study to analyse the prognostic power of MOF after ROSC in paediatric CA. Multiple organ failure was the main cause of death in 35.3% of the patients in our study. The scores in the PELOD and P-MODS MOF scales at 24–48h and five to seven days were significantly associated with mortality, which confirms the relevance of MOF in predicting the outcomes of children that achieve ROSC after CA.
The scores on the four scales were higher at 24–48h from admission compared to five to seven days. This probably reflects the greater initial severity after ROSC following CA. But we also need to take into account that some of the most severely ill patients, who logically would have higher scores early after ROSC, died in the first few days and were not assessed between days five and seven.
A study conducted in adults found that 66% of patients that had ROSC after in-hospital CA developed MOF, and that the most common organ failures, which also carry the worst prognosis, were cardiovascular and respiratory.3
In our study, ventilator peak inspiratory flow rates at 24–48h from CA were higher in patients that died. However, we did not identify any factors associated with increased mortality at five to seven days.
Limitations of the studyThere are limitations to our study. It is a single-centre retrospective study with a relatively small sample size, and thus prospective multicentric studies are required to confirm its findings. In addition, our study only assessed patients at two times: in the first 24–48h and five to seven days post CA. However, of all patients that died, the largest percentage corresponded to those that died after seven days, of who 50% died due to MOF. Therefore, future studies should evaluate patients after seven days post CA to better assess the clinical course of these patients.
ConclusionsThe mortality of children that recover from CA is high. The development of MOF following recovery from in-hospital CA in children is associated with increased mortality.
Further research is required to identify the organ systems that are most likely to be injured following ROSC to develop new therapeutic approaches aimed at reducing multiple organ injury and improving patient prognosis.
Conflict of interestsThe authors have no conflict of interests to declare.
Red de Salud Maternoinfantil y del Desarrollo (Mother and Child Health and Development Network, RedSAMID). RETICS funded by the 2008–2011 National Plan for R&D&I of Spain, Instituto de Salud Carlos III—General Subdirectorate of Research Evaluation and Promotion, European Regional Development Fund (ERDF), reference RD12/0026.
Please cite this article as: Carbayo T, de la Mata A, Sánchez M, López-Herce J, del Castillo J, Carrillo A, et al. Fallo multiorgánico tras la recuperación de la circulación espontánea en la parada cardiaca en el niño. An Pediatr (Barc). 2017;87:34–41.