An evaluation is made of the impact of a series of five interventions on the incidence of hospital-related infections in a level iii neonatal unit.
Material and methodsQuasi-experimental, pre-post intervention study, which included preterm infants weighing 1500g at birth or delivered at <32 weeks gestation, admitted in the 12 months before and after the measures were implemented (January 2014). The measures consisted of: optimising hand washing, following a protocol for insertion and handling of central intravenous catheters, encouraging breastfeeding; applying a protocol for rational antibiotic use, and establishing a surveillance system for multi-resistant bacteria. The primary endpoint was to assess the incidence of hospital-acquired infections before and after implementing the interventions.
ResultsThirty-three matched patients were included in each period. There was an incidence of 8.7 and 2.7 hospital-related infections/1000 hospital stay days in the pre- and post-intervention periods, respectively (P<.05). Additionally, patients in the treatment group showed a statistically-significant decrease in days on mechanical ventilation, use of blood products, and vasoactive drugs.
ConclusionsThe strategy, based on implementing five specific measures in a unit with a high rate of hospital-related infections, proved effective in reducing their incidence. This reduction could contribute to lowering the use of mechanical ventilation, blood products, and vasoactive drugs.
El objetivo de este estudio fue evaluar el impacto de un conjunto de 5 intervenciones sobre la incidencia de infecciones relacionadas con la asistencia sanitaria en una unidad de Neonatología de nivel iii.
Material y métodosEstudio cuasiexperimental pre-postintervención. Se incluyó a aquellos prematuros con peso al nacimiento<1.500g o edad gestacional<32 semanas que ingresaron en los 12 meses previos y posteriores a la implantación de las medidas (enero del 2014). Las intervenciones consistieron en optimizar la higiene de manos, protocolizar la inserción y la manipulación de catéteres intravenosos centrales, fomentar la alimentación con leche materna, implantar una política de uso racional de antibióticos y establecer un sistema de vigilancia epidemiológica de gérmenes multirresistentes. Como variable principal se analizó la densidad de incidencia de infecciones relacionadas con la asistencia sanitaria antes y después de implementar las medidas.
ResultadosFueron incluidos 33 pacientes en cada período, homogéneos en edad gestacional, peso y otras variables demográficas. Se constató una densidad de incidencia de 8,7 y 2,7 infecciones/1.000 días de estancia en los períodos pre y postintervención respectivamente (p<0,05). También se halló una disminución estadísticamente significativa en el porcentaje de días en ventilación mecánica, así como de pacientes que recibieron hemoderivados y fármacos vasoactivos.
ConclusionesEsta estrategia, basada en la puesta en marcha de 5 medidas concretas, fue efectiva en la disminución de infecciones relacionadas con la asistencia sanitaria en una unidad con tasas elevadas de dichas infecciones. Esta reducción pudo contribuir a una menor tasa de empleo de ventilación mecánica, hemoderivados y fármacos vasoactivos en el período postintervención.
Nosocomial infections are one of the main challenges we face in the management of very preterm newborns in neonatal intensive care units (NICUs), and it is one of the leading causes of morbidity and mortality in this population. The World Health Organization estimates that there are 4 million neonatal deaths a year, of which one third are due to severe infection.1
The incidence of nosocomial infection is higher in patients with birth weights of less than 1500g, and mortality is higher in this population.2 On the other hand, the indiscriminate use of antibiotics can promote infection by drug-resistant pathogens that are associated with higher mortality rates.3 Effective strategies to prevent nosocomial infections in NICUs need to be established.4
The risk factors for the development of nosocomial infections include those intrinsic to the patient, such as gestational age and weight at birth, genetic predisposition, the permeability of skin and mucosal barriers, male sex and immunosuppression,5 and extrinsic factors related to the interventions implemented in the NICU.
Chief among the extrinsic factors is the use of external devices, such as ventilatory support devices and especially central venous catheters5,6 and the administration of parenteral nutrition.7 The other key factor concerns human resources. Poor adherence with hand hygiene measures and inappropriate nurse-to-patient ratios are associated with an increase in the incidence of nosocomial infections and outbreaks of infection by multidrug-resistant pathogens.8
Other extrinsic factors associated with increases in the incidence of nosocomial infection are delayed initiation of enteral feeding, administration of ranitidine, prolonged antibiotic treatment and postnatal steroids use.5,9
The literature on the prevention of nosocomial infection is extensive. Some of the proposed measures are hand hygiene,10 aseptic technique in the handling of intravenous catheters,11 promoting breastfeeding,12 limiting the use of antibiotics13 and monitoring colonisation by multidrug-resistance microbes.8
We present the findings of a study based on a protocol that introduced 5 measures to decrease the incidence of nosocomial infection in very preterm and very low birth weight infants.
Materials and methodsThe primary objective of the study was to assess the impact of a bundle of 5 interventions, implemented over a period of 12 months, on the incidence of nosocomial infections in the population of very preterm and very-low-birth-weight infants.
Sample and methodsStudy designWe conducted a quasiexperimental pre-post intervention study in a III-B level NICU.
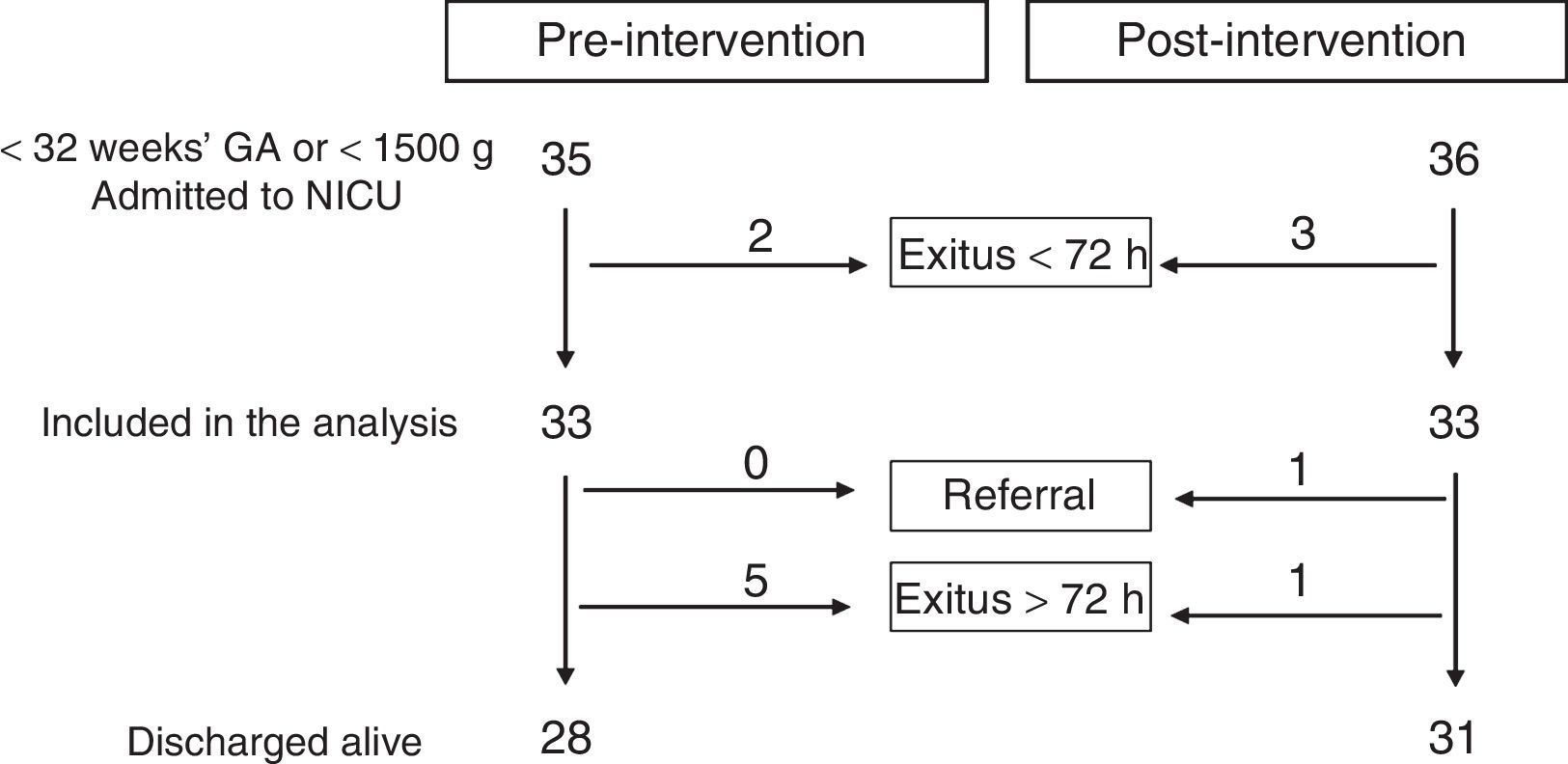
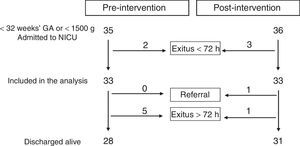
Study participantsThe study included preterm infants born with weights of less than 1500g or before 32 weeks gestation admitted to the NICU between January 2013 and January 2014 (pre-intervention period) and between January 2014 and January 2015 (post-intervention period). We excluded infants that died in the first 72h post birth.
DefinitionsNosocomial infectionWe considered the following nosocomial infections: episodes of late-onset neonatal sepsis (LONS), ventilator-associated pneumonia (VAP) and episodes of necrotising enterocolitis (NEC).
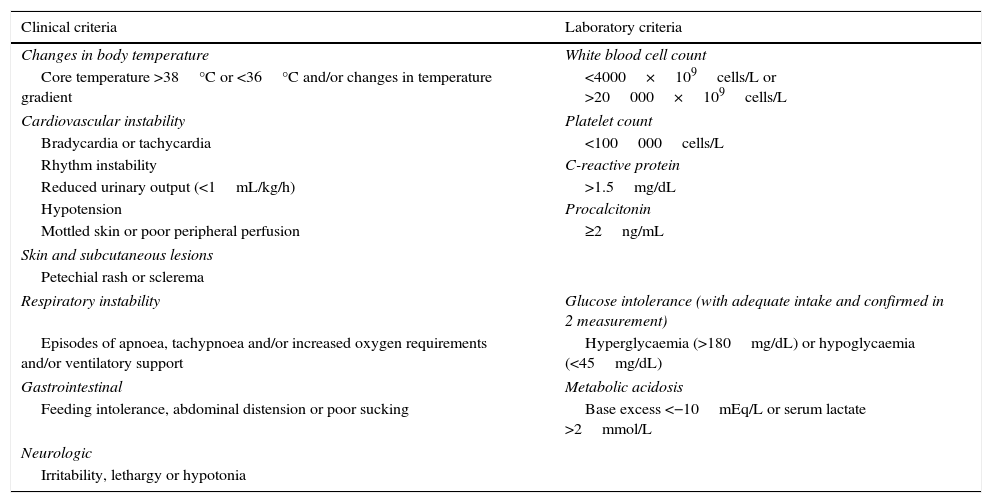
We defined LONS as the presence of 2 clinical signs and 2 laboratory signs indicative of systemic infection (Table 1) in patients with a postnatal age of more than 72h. We defined confirmed LONS as those cases in which the causative pathogen was isolated from blood culture, and probably LONS as cases with negative results of blood culture.
Clinical and laboratory criteria in the definition of late-onset neonatal sepsis.
| Clinical criteria | Laboratory criteria |
|---|---|
| Changes in body temperature | White blood cell count |
| Core temperature >38°C or <36°C and/or changes in temperature gradient | <4000×109cells/L or >20000×109cells/L |
| Cardiovascular instability | Platelet count |
| Bradycardia or tachycardia | <100000cells/L |
| Rhythm instability | C-reactive protein |
| Reduced urinary output (<1mL/kg/h) | >1.5mg/dL |
| Hypotension | Procalcitonin |
| Mottled skin or poor peripheral perfusion | ≥2ng/mL |
| Skin and subcutaneous lesions | |
| Petechial rash or sclerema | |
| Respiratory instability | Glucose intolerance (with adequate intake and confirmed in 2 measurement) |
| Episodes of apnoea, tachypnoea and/or increased oxygen requirements and/or ventilatory support | Hyperglycaemia (>180mg/dL) or hypoglycaemia (<45mg/dL) |
| Gastrointestinal | Metabolic acidosis |
| Feeding intolerance, abdominal distension or poor sucking | Base excess <−10mEq/L or serum lactate >2mmol/L |
| Neurologic | |
| Irritability, lethargy or hypotonia | |
Adapted from Rossi and Botgros.28
We defined catheter-related sepsis (CRS) as cases of LONS with a positive peripheral blood culture with recovery of the same pathogen (same organism and same antibiotic susceptibility profile) from catheter tip culture. We also classified bacteraemias that could not be attributed to another infectious source and developed in patients with an intravascular catheter as cases of CRS.14
We defined cases of VAP based on the fulfilment of these 3 criteria (22): 1) compatible radiological findings; 2) respiratory compromise, and 3) at least 4 of the following signs and symptoms: temperature instability, bradycardia, tachycardia, apnoea, tachypnoea, respiratory distress, respiratory secretions, C-reactive protein level >2mg/dL, immature/total neutrophil ratio >0.2.
We defined NEC as the presence of at least 2 compatible clinical manifestations with at least 1 of the following: pneumoperitoneum, pneumatosis intestinalis, unchanging rigid loops of small bowel.15
We defined outbreak of infection by a multidrug-resistant bacterium as recovery of the same bacterium in 2 or more patients within a period of less than 2 weeks, with evidence of resistance to 3 or more antibiotics to which the isolated bacterium is usually sensitive, such as beta-lactam antibiotics, carbapenems, aminoglycosides or quinolones.16,17
Variables under studyPrincipal variableWe assessed the impact of the interventions by comparing incidence rates, defined as the number of episodes of nosocomial infection per 1000 NICU patient days. We also compared the proportion of very preterm infants that had at least one episode of nosocomial infection during their stay in the NICU.
We calculated the proportion of CRS, LONS of non-catheter origin, VAP and NEC cases relative to the total number of nosocomial infections for each period. We conducted a separate analysis to compare the incidence rate of CRS (defined as the number of cases of CRS per 1000 days of central catheterization) in both periods.
Other variables of interestWe measured antibiotic use as the percentage of patient days in which patients were receiving some form of systemic antibiotherapy. We measured the total days of antibiotherapy as well as the days of empirical antibiotherapy. We quantified empirical antibiotherapy as the days of antibiotic treatment in the absence of confirmation of infection. The use of human milk was assessed by measuring the percentage of patients that were exclusively or partially fed breast milk between days 14 and 28 post birth.
We collected other data of interest to our study from the medical records of the patients, such as general demographic data and other clinical information.
Study protocol and data collectionDescription of interventionsBetween September 2013 and December 2013, we conducted a literature review to select the interventions to be implemented. We developed a timeline with deadlines for the design of interventions, the development of protocols and recording videos. In January 2014, we informed the staff and introduced the study protocol.
- 1.
Hand washing and hygiene policy. The entire staff participated in training sessions that reviewed the “five moments for hand hygiene” proposed by the World Health Organization, the “six steps for hand hygiene” and the use of alcohol-based disinfectant gels.10,18
- 2.
Aseptic technique protocol for the placement and management of central catheters. The protocol was developed by consensus and summarised aspects related to the insertion of central catheters, the maintenance of dressings, insertion site monitoring, and aseptic technique in the preparation of medication, the handling and maintenance of infusion devices and connectors, and the removal of central lines. Three videos that summarised the peripheral insertion of central access lines and their handling were recorded and distributed. We developed and introduced checklists to be implemented by the nurse in charge of the patient. Guidelines were developed for the early removal of central catheters.
- 3.
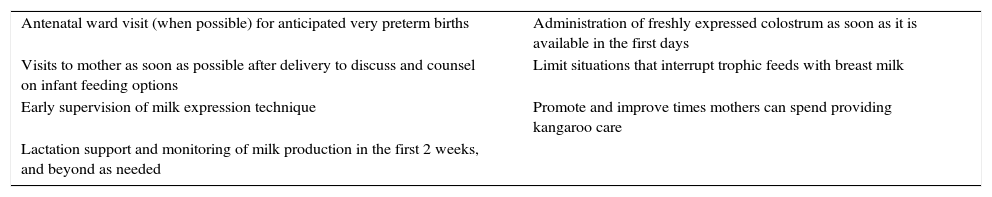
Initiatives to promote breastfeeding. We introduced a set of interventions aimed at increasing breastfeeding rates (Table 2).19 Educational workshops that reviewed different aspects related to breastfeeding were held in 2014.
Table 2.Interventions to increase breastfeeding rates.
Antenatal ward visit (when possible) for anticipated very preterm births Administration of freshly expressed colostrum as soon as it is available in the first days Visits to mother as soon as possible after delivery to discuss and counsel on infant feeding options Limit situations that interrupt trophic feeds with breast milk Early supervision of milk expression technique Promote and improve times mothers can spend providing kangaroo care Lactation support and monitoring of milk production in the first 2 weeks, and beyond as needed Adapted from Smith and Embleton.19
- 4.
Antimicrobial stewardship policy. A protocol was developed to minimise exposure to antibiotics whose key aspects were: 1) more stringent criteria for initiating antibiotherapy, omitting it in very preterm infants that are asymptomatic and have a low risk of infection, in whom the sole indication is the presence of a central venous line, or admitted for elevation of acute phase reactants with no associated symptoms; 2) early discontinuation of antibiotics initiated as empirical therapy or for suspected infection if blood culture results are negative at 48h from seeding and in the absence of clinical manifestations, and 3) adjusting antibiotic therapy schemes to use the minimum possible number of antibiotics and with the narrowest possible spectrum based on susceptibility testing results.20
- 5.
Epidemiological surveillance of multidrug-resistance bacteria. We established a surveillance system in close collaboration with the unit for the control of nosocomial infections of our hospital, performing monthly screenings of all patients admitted to the neonatal unit by means of rectal swab culture. We wrote a protocol for the isolation of patients with carrier status or active infection by multidrug-resistant organisms and for outbreak control.
We performed the statistical analysis using the statistical software SPSS version 17. We have expressed quantitative variables as medians, and categorical variables as absolute frequencies and proportions. We compared groups by means of the Mann–Whitney U test for quantitative variables, or Pearson's chi-squared test for qualitative variables. We defined statistical significance as a two-tailed P-value of less than .05. The values of variables measured in days were rounded to the nearest whole number.
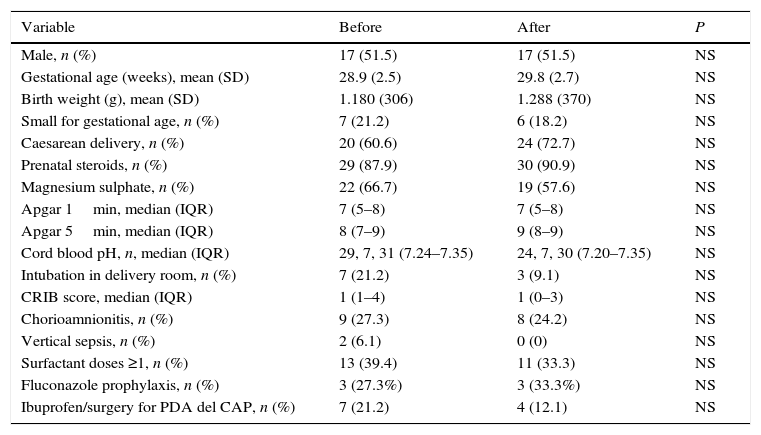
ResultsA total of 33 patients were included in each of the periods under study. Fig. 1 presents the flow chart of patient inclusion. Patients in both groups were comparable in terms of the various demographic variables under analysis (Table 3).
Demographic variables and comparative analysis of pre- and post-intervention periods.
| Variable | Before | After | P |
|---|---|---|---|
| Male, n (%) | 17 (51.5) | 17 (51.5) | NS |
| Gestational age (weeks), mean (SD) | 28.9 (2.5) | 29.8 (2.7) | NS |
| Birth weight (g), mean (SD) | 1.180 (306) | 1.288 (370) | NS |
| Small for gestational age, n (%) | 7 (21.2) | 6 (18.2) | NS |
| Caesarean delivery, n (%) | 20 (60.6) | 24 (72.7) | NS |
| Prenatal steroids, n (%) | 29 (87.9) | 30 (90.9) | NS |
| Magnesium sulphate, n (%) | 22 (66.7) | 19 (57.6) | NS |
| Apgar 1min, median (IQR) | 7 (5–8) | 7 (5–8) | NS |
| Apgar 5min, median (IQR) | 8 (7–9) | 9 (8–9) | NS |
| Cord blood pH, n, median (IQR) | 29, 7, 31 (7.24–7.35) | 24, 7, 30 (7.20–7.35) | NS |
| Intubation in delivery room, n (%) | 7 (21.2) | 3 (9.1) | NS |
| CRIB score, median (IQR) | 1 (1–4) | 1 (0–3) | NS |
| Chorioamnionitis, n (%) | 9 (27.3) | 8 (24.2) | NS |
| Vertical sepsis, n (%) | 2 (6.1) | 0 (0) | NS |
| Surfactant doses ≥1, n (%) | 13 (39.4) | 11 (33.3) | NS |
| Fluconazole prophylaxis, n (%) | 3 (27.3%) | 3 (33.3%) | NS |
| Ibuprofen/surgery for PDA del CAP, n (%) | 7 (21.2) | 4 (12.1) | NS |
IQR, interquartile range; PDA, patent ductus arteriosus.
A total of 18 nosocomial infection episodes were diagnosed, 14 in the pre-intervention period and 4 in the post-intervention period. The distribution by type of infection was: CRS, 7 (50%) pre-intervention and 3 (75.0%) post-intervention; non-catheter related sepsis, 3 (28.6%) in the pre-period and 0 in the post-period; NEC, 2 (14.3%) in the pre-period and 1 (25.0%) in the post-period; VAP, 1 (7.1%) in the pre-period and 0 in the post-period. The results of blood culture were positive in 15 out of the 18 episodes (83.3%). The isolated bacteria were Staphylococcus epidermidis (S. epidermidis) in 8 cases (53.3%), Klebsiella pneumoniae (K. pneumoniae) in 3 (20.0%), Enterococcus faecalis in 2 (13.3%), Candida albicans in 3 (20.0%) and Enterobacter cloacae in 1 (6.6%). More than one bacterium was isolated from blood culture in 2 episodes. The 3 episodes in which no bacteria were recovered from culture corresponded to 2 episodes of NEC and an episode of suspected non-catheter related sepsis.
We observed a statistically significant reduction in the incidence rate of nosocomial infection between the two periods, with a rate of 8.7 nosocomial infections/1000 patient days pre-intervention and a rate of 2.7 nosocomial infections/1000 patient days post-intervention (P<.05). The number of patients with more than 1 episode of nosocomial infection during their stay was 10 (30.3%) in the pre-intervention period and 4 (12.1%) in the post-intervention period, a result that tended towards statistical significance (P=.07).
There was a statistically significant reduction in the number of central catheter days per patient, with a median of 13.0 (IQR, 8.0–17.5) in the pre-intervention period and of 9.0 (IQR, 0–15.0) in the post-intervention period (P<.05). In 6 out of the 10 episodes of CRS (54.5%), the same organism was recovered from the blood culture and the catheter tip culture (S. epidermidis in 3 cases, C. parapsilosis and K. pneumoniae). In 4 episodes (45.5%), sepsis was attributed to the indwelling catheter but the same organism was not recovered from the tip culture. We observed a reduction in the number of CRS episodes/1000 central catheter days from 14.4 in the pre-intervention period to 9.6 in the post-intervention period, although the difference was not statistically significant (P=.54).
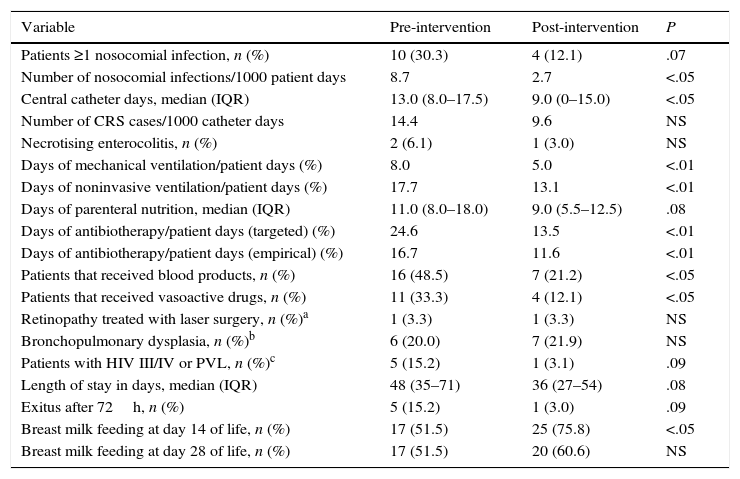
Table 4 summarises the findings for the rest of the secondary variables under analysis. The most salient findings were statistically significant decreases in the proportion of days of mechanical ventilation during the stay (both invasive and noninvasive) and in the proportion of patients that received vasoactive drugs and blood product transfusions. We found decreases in length of stay, days of parenteral nutrition and the proportion of patients that died or had severe lesions in neuroimaging that approximated statistical significance.
Outcome variables and comparative analysis of the pre- and post-intervention periods.
| Variable | Pre-intervention | Post-intervention | P |
|---|---|---|---|
| Patients ≥1 nosocomial infection, n (%) | 10 (30.3) | 4 (12.1) | .07 |
| Number of nosocomial infections/1000 patient days | 8.7 | 2.7 | <.05 |
| Central catheter days, median (IQR) | 13.0 (8.0–17.5) | 9.0 (0–15.0) | <.05 |
| Number of CRS cases/1000 catheter days | 14.4 | 9.6 | NS |
| Necrotising enterocolitis, n (%) | 2 (6.1) | 1 (3.0) | NS |
| Days of mechanical ventilation/patient days (%) | 8.0 | 5.0 | <.01 |
| Days of noninvasive ventilation/patient days (%) | 17.7 | 13.1 | <.01 |
| Days of parenteral nutrition, median (IQR) | 11.0 (8.0–18.0) | 9.0 (5.5–12.5) | .08 |
| Days of antibiotherapy/patient days (targeted) (%) | 24.6 | 13.5 | <.01 |
| Days of antibiotherapy/patient days (empirical) (%) | 16.7 | 11.6 | <.01 |
| Patients that received blood products, n (%) | 16 (48.5) | 7 (21.2) | <.05 |
| Patients that received vasoactive drugs, n (%) | 11 (33.3) | 4 (12.1) | <.05 |
| Retinopathy treated with laser surgery, n (%)a | 1 (3.3) | 1 (3.3) | NS |
| Bronchopulmonary dysplasia, n (%)b | 6 (20.0) | 7 (21.9) | NS |
| Patients with HIV III/IV or PVL, n (%)c | 5 (15.2) | 1 (3.1) | .09 |
| Length of stay in days, median (IQR) | 48 (35–71) | 36 (27–54) | .08 |
| Exitus after 72h, n (%) | 5 (15.2) | 1 (3.0) | .09 |
| Breast milk feeding at day 14 of life, n (%) | 17 (51.5) | 25 (75.8) | <.05 |
| Breast milk feeding at day 28 of life, n (%) | 17 (51.5) | 20 (60.6) | NS |
CRS, catheter-related sepsis; IQR, interquartile range; IVH, intraventricular haemorrhage; PVL, periventricular leukomalacia.
We observed a statistically significant reduction in the number of days of antibiotherapy relative to patient days (Table 4). This reduction was sustained in the separate analysis of the days of empirical antibiotherapy. The proportion of patients that received human milk between days 14 and 28 of life was higher in the post-intervention period, and the difference was statistically significant for day 14 post birth.
During the pre-intervention period, there were 3 outbreaks by multidrug-resistant organisms (OXA1-type beta-lactamase-producing K. pneumoniae and Serratia marcescens in 2 occasions). These outbreaks caused 3 cases of 3 sepsis and 10 local infections (9 cases of conjunctivitis and 1 case of surgical site infection). There were no cases of systemic infection by multidrug-resistant organisms in the post-intervention period. The screening conducted in February 2016 identified 2 patients that were asymptomatic carriers of broad-spectrum beta-lactamase-producing K. pneumoniae. These patients were isolated, and colonisation by this organism was not identified in any further patients. The rest of the screens in the post-intervention period were negative.
DiscussionDespite the numerous interventions that have been proven useful in the prevention of nosocomial infections, the latter continues to be one of the main problems in neonatal care units.7,21 There is wide variability in the incidence of nosocomial infection between hospitals,5 and every facility needs to develop preventive strategies suitable to its own circumstances.21
In January 2013, our unit became a IIIB level unit (from being a IIIA unit). After the first 12 months of offering care for very preterm infants, we reviewed different quality assessment indicators in our unit. The difference in the incidence of nosocomial infection compared to the one reported in the literature22 (8.7 vs 6.4 nosocomial infections/1000 patient days) motivated us to design a control cycle to improve care. The intervention included 5 evidence-based strategies adapted to our needs. The implementation of these measures achieved an incidence rate within the established quality standards (2.7 nosocomial infections/1000 patient days).
Catheter-related sepsis was the most frequent type of sepsis in both periods under study. The introduction of specific protocols for the insertion and maintenance of central catheters has been shown to reduce the incidence of CRS.11 Different institutions and authors have published reviews and clinical practice guidelines based on current scientific evidence to achieve this goal.4 Our outcomes corresponded to an incidence rate that exceeded those reported in other series22 (14.4 vs 11.1 cases of CRS/1000 catheter days). The new protocol for the insertion and handling of central catheters succeeded in reducing this rate by nearly 25% (to 9.6 CRS cases/1000 catheter days).
Many studies have demonstrated the protective effect of breastfeeding against NEC and late-onset neonatal sepsis.12 There is evidence of a dose-response relationship in this effect between days 1 and 28 post birth.23,24 Breast milk feeding has also been proposed as a protective factor against colonisation by multidrug-resistant bacteria.25 We believed that we could improve the percentage of patients that were fed breast milk, and the efforts devoted to the purpose increased this percentage by more than 50%. In the only case of NEC diagnosed during the post-intervention period, the patient could not be fed breast milk because the mother had a disease that could be transmitted through breast milk and we could not provide donor milk, as our hospital did not have a human milk bank or cooperate with one.
Among the strategies described in the literature, hand hygiene constitutes another cornerstone in the prevention of nosocomial infections, as it prevents colonisation of the skin, airways and gastrointestinal tract by potentially pathogenic organisms. These organisms are disseminated from patient to patient through the hands of health care staff.4,8 Training and awareness campaigns on correct handwashing and adherence to the use of alcohol-based antimicrobial gels aimed at health care staff are effective in reducing colonisation by multidrug-resistant organisms.4,26 Correct hand hygiene prevents outbreaks of nosocomial infection by this type of pathogen, which have even led to the closure of some neonatal care units.16 During the pre-intervention period, there were 3 outbreaks by multidrug-resistant bacteria, a situation that did not recur once the preventive measures were implemented.
The use of antibiotics is widespread in NICUs, and 47% of patients admitted to NICUs do receive them.20 Preterm infants that have long courses of empirical antibiotherapy (>5 days) with a sterile blood culture are at higher risk of sepsis, enterocolitis and death, as well as increased neurologic sequelae.13 Thanks to the antimicrobial stewardship protocol, the percentage of days of empirical antibiotherapy in the post-intervention period decreased by 30%. This result probably contributed to the in nosocomial infection and colonisation by multidrug-resistant organisms.
During the second period, the reduction in the incidence of nosocomial infection was accompanied by a decreased mortality that tended towards statistical significance. We also observed a decrease in other illnesses (assessed by the use of blood products, vasoactive drugs and days of mechanical ventilation). Furthermore, the proportion of patients with lesions detected by neuroimaging decreased by a factor of 5. Therefore, these strategies and their results may have benefits in psychomotor development at later stages in life.
The reduction in the number of nosocomial infections and other associated comorbidities entails a reduction in health care costs. It has been estimated that episodes of LONS in very preterm infants result in a 15% increase in the cost of their care.27 In the post-intervention period, we observed a median reduction in length of stay of 25%, which a probability that neared statistically significance.
The weaknesses of our study derive from the small sample size. The number of patients included per period allowed us to identify statistically significant differences in the primary outcome variable, however, the analysis of secondary outcomes was limited due to this reason.
In conclusion, the strategies introduced in our hospital were efficacious in reducing the incidence of nosocomial infection. Our experience may be useful for units which, as was our case, have rates that exceed the limit established by quality standards.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García González A, Leante Castellanos JL, Fuentes Gutiérrez C, Lloreda García JM, Fernández Fructuoso JR, Gómez Santos E, et al. Cinco pasos para la disminución de las infecciones relacionadas con la asistencia sanitaria en prematuros de muy bajo peso. Estudio cuasiexperimental. An Pediatr (Barc). 2017;87:26–33.
Previous presentations: This study was presented at the XXV Congreso de Neonatología, May 20–22, 2015; Seville, Spain.