Recent studies have shown changes in the aetiology of serious bacterial infections in febrile infants ≤90 days of age. The aim of this study was to describe the current microbiology and outcomes of these infections in Spain.
Material and methodsSub-analysis of a prospective multicentre study focusing on febrile infants of less than 91 days of life, admitted between October 2011 and September 2013 to Emergency Departments of 19 Spanish hospitals, members of the Spanish Paediatric Emergency Research Group of the Spanish Society of Paediatric Emergencies (RISeuP/SPERG).
ResultsThe analysis included 3401 febrile infants ≤90 days of age with fever without source. There were 896 positive cultures: 766 urine (85.5%), 100 blood (11.2%), 18 cerebrospinal fluid (2%), 10 stool, and 2 umbilical cultures. Among the 3401 infants included, 784 (23%) were diagnosed with a serious bacterial infection, and 107 of them (3.1%) with an invasive infection.
E. coli was the most common pathogen isolated from urine (628; 82%), blood (46; 46%), and cerebrospinal fluid cultures (7; 38.9%), followed by S. agalactiae that was isolated from 24 (24%) blood cultures and 3 (16.7%) cerebrospinal fluid cultures. There were only 2 L. monocytogenes infections. Four children died, and seven had severe complications.
ConclusionsAmong infants ≤90 days of age with fever without source, E. coli was the most common pathogen isolated from urine, blood, and cerebrospinal fluid cultures.
Estudios recientes han demostrado cambios en la etiología de las infecciones bacterianas potencialmente graves en lactantes menores de 3 meses de vida con fiebre. El objetivo es describir la microbiología y la evolución de estas infecciones en nuestro entorno.
Material y métodosSubanálisis de un estudio prospectivo y multicéntrico sobre lactantes febriles con menos de 3 meses de edad que consultaron desde el 1 de octubre de 2011 hasta el 30 de septiembre de 2013 en los servicios de urgencias de 19 hospitales infantiles españoles de la Red de investigación de la Sociedad Española de Urgencias de Pediatría/Spanish Pediatric Emergency Research Group (RISeuP/SPERG).
ResultadosSe incluyó a 3.401 lactantes menores de 91 días de vida con fiebre sin foco. Hubo 896 cultivos positivos: 766 urocultivos (85,5%), 100 hemocultivos (11,2%), 18 cultivos de líquido cefalorraquídeo (2%), 10 coprocultivos y 2 cultivos umbilicales. Fueron diagnosticados de una infección bacteriana potencialmente grave 784 niños (23%), de los cuales 107 (3,1%) tenían una infección invasora.
Escherichia coli (E. coli) fue la bacteria más frecuente de urocultivos (628; 82%), hemocultivos (46; 46%) y cultivos de líquido cefalorraquídeo (7; 38,9%) seguido por Streptococcus agalactiae, que fue aislado en 24 (24%) hemocultivos y 3 (16,7%) cultivos de líquido cefalorraquídeo. Solo hubo 2 infecciones producidas por Listeria monocytogenes. Fallecieron 4 niños y 7 desarrollaron complicaciones graves.
ConclusionesE. coli fue la bacteria más frecuente en urocultivos, hemocultivos y cultivos de líquido cefalorraquídeo de los lactantes con menos de 3 meses de vida y fiebre sin foco, incluso entre los neonatos.
Potentially serious bacterial infections (PSBIs) in febrile infants aged less than 3 months are more frequent, carry a poorer prognosis and have a different aetiology compared to older children. Streptococcus agalactiae (S. agalactiae, historically the leading causative agent of bacteraemia and meningitis in infants <3 months), gram-negative rods, especially Escherichia coli (E. coli), Listeria monocytogenes (L. monocytogenes) and Enterococcus species are the agents typically involved in PSBIs in this age group. With the exception of E. coli, which continues to be the aetiological agent most frequently involved in urinary tract infections throughout childhood, the prevalence of infection by these microorganisms is low in older children.1–7 All of the above warrants the performance of diagnostic tests and hospital admission with antibiotic therapy (ampicillin and an aminoglycoside or a third-generation cephalosporin to cover the bacteria detailed above) in the management of all febrile infants aged less than 3 months with risk factors.6–9
A study of febrile infants aged less than 3 months that received care in a Spanish emergency department over a period of 5 years described changes in the aetiology of bacteraemia; E. coli was the most prevalent bacterium and there were no cases of infection by L. monocytogenes.10 Recent studies in the United States have also shown that the incidence of bacteraemia and meningitis caused by S. agalactiae is decreasing, so that E. coli is currently the most prevalent pathogen not only in urinary tract infections, but also in cases of bacteraemia and meningitis in infants aged less than 3 months.11–14
The main objective of this study was to describe the microbiology and outcomes of infants aged less than 91 days with fever without source (FWS) that received a diagnosis of PSBI in Spain.
Materials and methodsWe performed a subanalysis in the framework of a prospective, multicentric study conducted between October 1, 2011 and September 30, 2013 in 19 Spanish Children's hospitals in 8 autonomous communities (Table 1) members of the Working Group on the Febrile Infant of the Spanish Pediatric Emergency Research Group of the Spanish Society of Pediatric Emergencies (RISeuP/SPERG.)
Participating hospitals, and number of patients included in the study.
| Autonomous community | Hospital | Number of patients |
|---|---|---|
| Andalucía | H. Virgen de las Nieves de Granada | 242 |
| H. Carlos Haya de Málaga | 440 | |
| Asturias | H. de Cabueñes de Gijón | 38 |
| Castilla y León | H. Río Hortega de Valladolid | 162 |
| Castilla-La Mancha | H. Virgen de la Salud de Toledo | 144 |
| Cataluña | H. Vall d’Hebron de Barcelona | 188 |
| Corporació Sanitària Parc Taulí de Sabadell | 84 | |
| H. de Nens de Barcelona | 105 | |
| H. Arnau de Vilanova de Lleida | 141 | |
| Euskadi | H. Cruces de Bilbao | 395 |
| H. de Basurto | 162 | |
| H. Alto Deba de Arrasate-Mondragón | 17 | |
| Madrid | H. Niño Jesús | 268 |
| H. Gregorio Marañón | 267 | |
| H. Fuenlabrada | 85 | |
| H. Infanta Sofía | 101 | |
| H. San Rafael | 100 | |
| Murcia | H. Virgen de la Arrixaca de Murcia | 403 |
| Valencia | H. Quirón de Valencia | 59 |
To be included in the study, infants aged less than 91 days had to meet the following criteria: seeking care for FWS, availability of the results of complete blood count, C-reactive protein test, blood culture, urine analysis (with urine test strip), and urine culture of a sample obtained by catheter, and signed informed consent by the parents or legal guardians.
We excluded patients whose temperature had been estimated at home without a thermometer and who were afebrile in the emergency department.
The study did not change the approach to the management of patients, which conformed to the established protocols of each hospital.
We collected the following data for each patient: age, sex, date of service, personal history, time elapsed from the detection of fever to receiving care at the emergency department, temperature at home and at the emergency department, general health status, results of diagnostic tests, final diagnosis and patient destination after discharge. The follow-up protocol included a telephone call within 30 days from the emergency department visit to gather information about the outcome of patients that were not admitted to hospital.
The principal investigator in each hospital registered patient data using a form created for the purpose in Google Drive®. The registers of all hospitals were consolidated into a single database (Microsoft Excel®) to which only principal investigators were given access. No personally identifiable data were entered in the database for any of the patients.
The study was approved by the Ethics Committee of the Hospital Infantil Universitario Niño Jesús de Madrid and, when required, by the institutional ethics committees of participating hospitals.
DefinitionsFever: axilla or rectal temperature ≥38°C at home or in the emergency department.
FWS: febrile illness whose cause remains undetermined after the history-taking and physical examination.
Previously healthy: born at term; not treated for unexplained neonatal jaundice, not hospitalzed longer than the mother, postnatal antibiotherapy, past hospitalisations or chronic underlying disease.
Well appearing: defined by a normal Paediatric Assessment Triangle in the emergency departments that used it, and for the other hospitals infants were considered well appearing if they presented with good colour, good peripheral perfusion, alert, responsive and without breathing difficulties.
Invasive bacterial infection (IBI): isolation of a pathogenic bacterium from cerebrospinal fluid (CSF), blood or any other normally sterile site (bone, joint, lymph node, pleural fluid, etc.). The presence of the following bacteria in immunocompetent patients was interpreted as contamination: Staphylococcus epidermidis, Propionibacterium acnes, Streptococcus viridans or diphtheroids.
Noninvasive bacterial infection: positive result of urine, stool or umbilical cord blood culture:
- –
Positive urine culture: any urine culture from a sample collected by sterile methods (urinary catheter) with more than 10000CFU/mL of a single bacterium. The following were considered to be true pathogens: E. coli, Klebsiella spp., Enterococcus spp, Proteus mirabilis, Citrobacter freundii, Enterobacter spp, Citrobacter koseri, Staphylococcus aureus (S. aureus) or S. agalactiae.
- –
Positive stool culture: growth in stool culture of Salmonella, Shigella spp, Campylobacter jejuni (C. jejuni) or Yersinia enterocolitica.
- –
Positive umbilical cord culture: isolation of a single bacterium (S. aureus, Streptococcus pyogenes, gram-negative bacilli).
We have expressed categorical variables as absolute frequencies and percentages. We have compared proportions by means of the chi-squared test and, for small samples, by Fisher's exact test. We considered tests with p-values of less than 0.05 statistically significant.
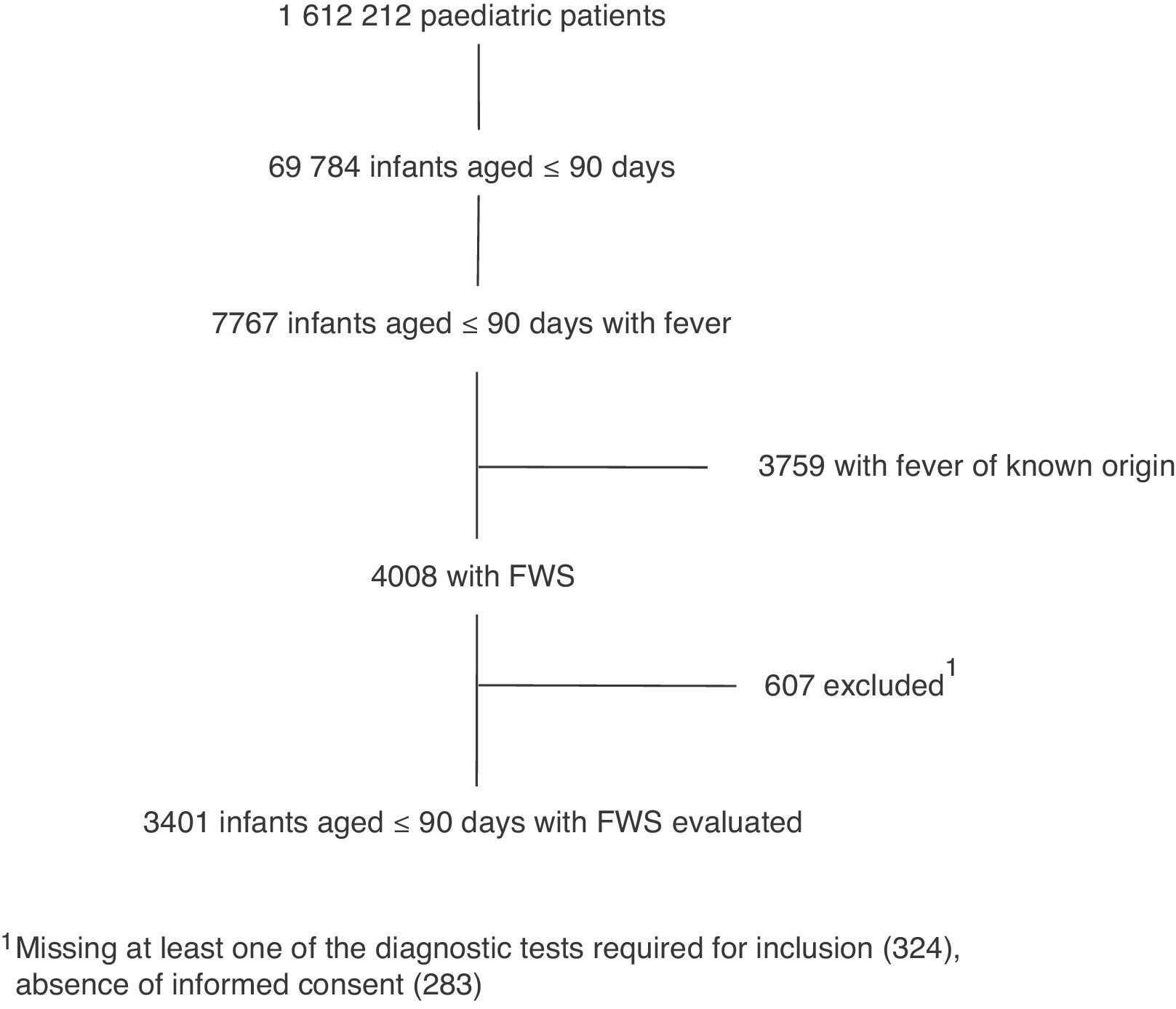
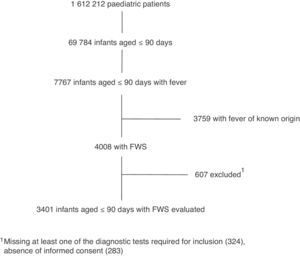
ResultsOver the 2-year study period, there were 1612212 visits to the 19 participating emergency departments, of which 4008 (0.25%) corresponded to infants aged less than 91 days with FWS. Finally, after applying the exclusion criteria, 3401 children were included in the study (Fig. 1) with the following age distribution: ≤28 days, 905 (26.6%), 29–59 days, 1404 (41.3%); 60–90 days, 1092 (32.1%).
Table 2 summarises the characteristics of the patients.
Characteristics of the 3401 patients included in the study.
| Boys/girls | 2029/1372 |
| Previously healthy | 2939 (86.4%) |
| Well appearing | 3034 (89.2%) |
| Complete sepsis evaluationa | 878 (25.8%) |
| ≤28 days | 549 (60.7%) |
| 29–59 days | 227 (16.2%) |
| 60–90 days | 102 (9.3%) |
| Hospitalized | 1836 (55.5%) |
| ≤28 days | 763 (87.6%) |
| 29–59 days | 676 (48.1%) |
| 60–90 days | 420 (38.5%) |
| Antibiotic treatment | 1464 (43%) |
| ≤28 days | 555 (61.3%) |
| 29–59 days | 499 (35%) |
| 60–90 days | 410 (37.5%) |
Among all the cultures done in the 3401 infants aged less than 91 days with FWS, 896 were positive: 766 urine cultures (85.5%), 100 blood cultures (11.2%), 18 CSF cultures (2%), 10 stool cultures (Salmonella in 6; C. jejuni in 4) and 2 umbilical cord cultures (E. coli in 2). A diagnosis of PSBI was made in 784 infants (23%), 107 (3.1%) of them had an IBI.
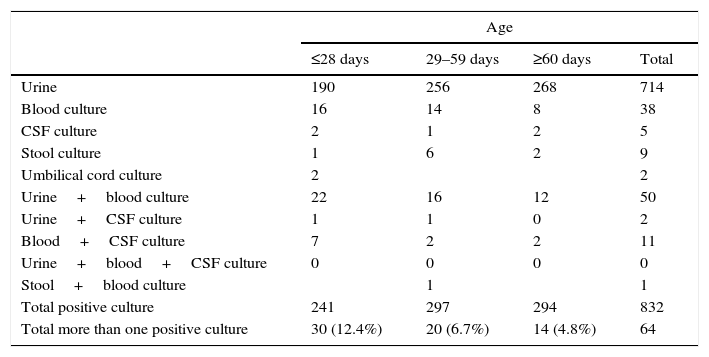
Bacteria were isolated from more than one type of culture in 64 patients (Table 3). A single bacterium was isolated in 44 of the children with positive blood and urine cultures (E. coli in 39 infants; and Klebsiella pneumoniae, Enterobacter cloacae, Enterococcus faecalis, S. agalactiae and S. aureus in one patient each), in all of the infants with positive blood and CSF cultures (E. coli 4; S. agalactiae 3; Streptococcus pneumoniae [S. pneumoniae] 2; Morganella morganii 1; Pasteurella multocida 1), in 1 infant with urine and CSF cultures positive for E. coli and in 1 infant with stool and blood cultures positive for C. jejuni.
Types of culture with positive results.
| Age | ||||
|---|---|---|---|---|
| ≤28 days | 29–59 days | ≥60 days | Total | |
| Urine | 190 | 256 | 268 | 714 |
| Blood culture | 16 | 14 | 8 | 38 |
| CSF culture | 2 | 1 | 2 | 5 |
| Stool culture | 1 | 6 | 2 | 9 |
| Umbilical cord culture | 2 | 2 | ||
| Urine+blood culture | 22 | 16 | 12 | 50 |
| Urine+CSF culture | 1 | 1 | 0 | 2 |
| Blood+CSF culture | 7 | 2 | 2 | 11 |
| Urine+blood+CSF culture | 0 | 0 | 0 | 0 |
| Stool+blood culture | 1 | 1 | ||
| Total positive culture | 241 | 297 | 294 | 832 |
| Total more than one positive culture | 30 (12.4%) | 20 (6.7%) | 14 (4.8%) | 64 |
The proportion of patients with 2 different positive cultures was greater in newborns (30/241; 12.4%) than in infants aged 29–59 days (20/297; 6.7%) or 60–90 days (14/294; 4.8%); differences that were statistically significant (p=0.003).
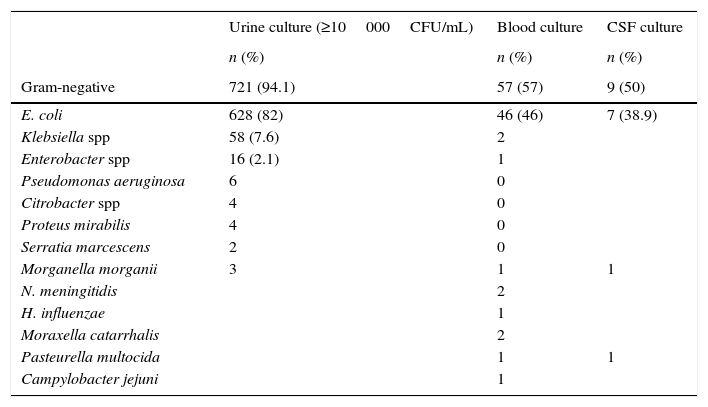
E. coli was the bacterium isolated most frequently in urine, blood and CSF cultures (Table 4).
Bacteria isolated in urine, blood and CSF cultures.
| Urine culture (≥10000CFU/mL) | Blood culture | CSF culture | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Gram-negative | 721 (94.1) | 57 (57) | 9 (50) |
| E. coli | 628 (82) | 46 (46) | 7 (38.9) |
| Klebsiella spp | 58 (7.6) | 2 | |
| Enterobacter spp | 16 (2.1) | 1 | |
| Pseudomonas aeruginosa | 6 | 0 | |
| Citrobacter spp | 4 | 0 | |
| Proteus mirabilis | 4 | 0 | |
| Serratia marcescens | 2 | 0 | |
| Morganella morganii | 3 | 1 | 1 |
| N. meningitidis | 2 | ||
| H. influenzae | 1 | ||
| Moraxella catarrhalis | 2 | ||
| Pasteurella multocida | 1 | 1 | |
| Campylobacter jejuni | 1 |
| Gram-positive | 45 (5.9) | 43 (43) | 9 (50) |
|---|---|---|---|
| Enterococcus spp | 37 (4.8) | 5 | |
| S. aureus | 5 | 7 (7) | 2 |
| S. agalactiae | 3 | 24 (24) | 3 (16.7) |
| S. pneumoniae | 6 (6) | 3 (16.7) | |
| L. monocytogenes | 1 | 1 | |
| Total | 766 | 100 | 18 |
In the subgroup of newborns (905 patients aged ≤28 days of life), in which there were 268 (29.6%) positive cultures (213 urine, 45 blood and 10 CSF cultures), the distribution of bacterial species was similar to the one found in the general population:
- –
Urine cultures: E. coli (171/213; 80.3%), Klebsiella spp (15/213; 7%) and Enterococcus spp (14/213; 6.6%). Two urine cultures were positive for S. agalactiae in 2 newborns aged more than 7 days, one of who had bacteraemia.
- –
Blood cultures: E. coli (25/45; 55.5%) and S. agalactiae (11/45; 24.4%; three of these patients were aged less than 8 days).
- –
CSF cultures: E. coli (6/10) and S. agalactiae (2/10; associated with bacteraemia in both patients).
Two cultures tested positive for L. monocytogenes: the blood culture of a male newborn that presented with a fever lasting 17h, feeding refusal, irritability and poor general appearance; and the CSF culture of a well appearing boy aged 36 days with fever lasting 12h.
There were 7 cases of IBI caused by S. pneumoniae (4 with positive blood culture, 1 with positive CSF culture, 2 with positive blood and CSF cultures), of which 2 corresponded to infants aged 29–59 days and 5 to infants aged more than 59 days.
Three patients received a diagnosis of herpes simplex infection. One was a well appearing girl aged 8 days that presented with fever lasting 5h with no associated symptoms, treated with acyclovir following the detection of herpes simplex type 1 by PCR from a CSF sample (traumatic lumbar puncture), who had a favourable outcome free from sequelae. Another corresponded to a previously healthy girl aged 7 days that presented with a fever lasting 3h, irritability and well general appearence and who got worse during the hospital stay and died 3 days after. The autopsy revealed an infection by herpes simplex virus type 1. The results of the lumbar puncture analysis had been normal. Another was a boy aged 61 days that presented with a fever lasting 12h, irritability and well general appearence and was admitted for antibiotherapy with a diagnosis of meningitis (77 white blood cells/mm3 in CSF); herpes simplex was detected by PCR and the patient was treated with acyclovir, in spite of which he developed severe neurologic sequelae (epilepsy, psychomotor impairment).
Four patients died, all of them had been admitted for intravenous antibiotherapy in their first visit. A girl aged 77 days, previously healthy, with fever lasting 12h, poor general appearence and a consolidation in the evinced by chest radiography. She was diagnosed with sepsis and died due to multiple organ failure. Bacterial culture results were negative. A girl aged 77 days with hypotonia on physical examination that presented with fever lasting 3h and in poor general appearance. She was admitted with respiratory distress and received a diagnosis of bronchiolitis. Bacterial culture results were negative. A previously healthy boy aged 32 days presenting with fever lasting 3h, poor general appearance and a purpuric rash. Neisseria meningitidis group B was isolated from the blood culture, and E. coli from the urine culture. Also, the girl aged 7 days mentioned above with a herpes simplex type 1 infection identified during the autopsy.
Another 7 patients had complications or serious sequelae: 3 patients aged 20, 58 and 71 days with bacterial meningitis caused by E. coli, S. agalactiae and S. pneumoniae, respectively, developed complications and severe and permanent neurologic sequelae; one boy aged 13 days with meningitis caused by enterovirus complicated with myocarditis; the boy aged 61 days with meningitis caused by herpes simplex described above; one boy aged 38 days with sepsis due to S. agalactiae associated with arthritis and left-shoulder myositis requiring surgical drainage; and one boy aged 66 days with left coronary artery ectasia secondary to Kawasaki disease.
DiscussionIn our study, E. coli was the bacterium involved most frequently in invasive and noninvasive PSBIs in febrile infants aged less than 3 months, which confirms that the epidemiology of PSBIs in this age group is changing; we also observed that the proportion of patients with urinary tract infections (UTIs) had increased considerably compared to the proportion of patients with bacteraemia or meningitis, a shift that has also been described in the United States.13,14 Another salient finding was that nearly 50% of positive blood cultures were associated with a UTI. These percentages are slightly above those reported by Gómez et al, who analysed 1018 blood cultures ordered for 1125 infants aged less than 3 months presenting with FWS in the emergency department of a tertiary care hospital in the Basque Country (2003–2008), and found that 8 (34.8%) of the 23 diagnosed cases of bacteraemia were associated with a UTI.10 Overall, these findings emphasise the importance of urine testing and urine culture in the assessment of infants aged less than 3 months presenting with FWS.
As is the case in other countries, E. coli has become the bacterium most frequently isolated from urine, blood and CSF cultures in infants aged less than 3 months presenting with FWS10–14 and, also in newborns. All of this may be a reflection of some of the preventive measures implemented in recent years that have led to decreases in the incidence of infections by S. agalactiae, L. monocytogenes and S. pneumoniae. Programmes for S. agalactiae screening during pregnancy with antibiotic treatment of positive cases at the time of delivery have been associated with a decreased incidence of early-onset neonatal sepsis (≤7 days of life)15–17; in our series, all but 3 of the cultures positive for S. agalactiae were in infants aged more than 7 days. L. monocytogenes is a gram-positive bacillus that can be vertically transmitted during pregnancy or acquired through the consumption of contaminated food.18,19 Our study seems to suggest that the educational campaign recommending pregnant women to avoid risky foods has been effective. Although the incidence of listeriosis in Spain has increased in the last decade (in 2012, Spain had the second largest number of reported cases of infection by L. monocytogenes in the European Union),19 in our series we only found 2 IBIs caused by this bacterium. The introduction of the pneumococcal conjugate has reduced the incidence of invasive disease in vaccinated as well as unvaccinated children,20–25 which may reflect, as Poehling et al. suggested,26 the benefits of herd immunity for infants that had yet to receive any doses due to their age. We should also mention that none of the 7 pneumococcal infectious were diagnosed in newborns.
The main goal of paediatricians that manage infants aged less than 3 months presenting with FWS is the early diagnosis and treatment of PSBIs and especially IBIs (bacteraemia and meningitis). We should highlight that in our study, 6 of the 11 patients that died or developed severe sequelae had a meningeal infection, but in 3 of them the aetiology was viral (2 herpes simplex and one enterovirus). It is important to remember that early treatment of herpes infections is a significant prognostic factor and that, while infrequent, they must be included in the differential diagnosis on account of the considerable associated morbidity. Some care guidelines for the management of FWS in infants aged less than 3 months already recommend considering this infection, especially in newborns, if the patient presents with cutaneous vesicles, seizures, elevated transaminases or critical illness.27
Last of all, this case series with patients aged less than 3 months presenting with FWS is fairly representative of the management of these patients in Spanish emergency departments. Despite the current protocols for the management of FWS,8,9 lumbar puncture and antibiotic treatment were only performed in 6 out of 10 newborns. It seems that clinicians prefer to adopt a watchful waiting approach, and nearly 90% of newborns with fever are admitted to hospital. These findings are similar to those recently published by Jain et al,28 who found that a full sepsis evaluation (blood, urine and CSF tests and culture) was performed in 73% of the 2253 febrile newborns that visited 36 emergency departments in the United States in 2010. As one would expect, the approach was even less aggressive in infants aged more than 28 days.
It is important that further studies on this subject be conducted in Spain in order to detect epidemiological changes, especially with the purpose of determining which is the most appropriate empirical antibiotic treatment for infants aged less than 3 months presenting with FWS that are not considered low-risk. Another interesting finding was that S. aureus was the third most frequent organism isolated from blood cultures, following E. coli and S. agalactiae. For the time being, there is no question that empirical treatment should include an aminoglycoside or a third-generation cephalosporin to cover gram-negative bacilli. Ampicillin should also be part of the initial treatment because L. monocytogenes, while rare, has not been eradicated, and also there are also still UTIs as well as cases of bacteraemia caused by Enterococcus spp, although we must be particularly mindful of changes in listeriosis and IBI caused by S. aureus.
There are two limitations to this study. The first is that it only included febrile infants, and did not take into account all infants with a positive culture, including those without fever. It is well known that infants aged less than 3 months may have PSBIs in the absence of fever, and in some series the proportion of febrile patients of this age with bacteraemia has reached up to 7%,10 but the original study was focused on patients with fever, not on positive cultures. Nevertheless, our study contributes relevant data on a well-defined population and, in fact, its findings are similar to those of studies that included all positive cultures in febrile and afebrile patients.11,13 The second limitation is that we did not gather data on antibiotic susceptibility, when knowledge of antibiotic resistance profiles in bacteria is essential to choose the most appropriate treatment.
To conclude, we would like to highlight that at present E. coli is the bacterium isolated most frequently in urine, blood and CSF cultures in infants aged less than 91 days with FWS in Spain, even if we only take newborns into account. In our country, the prevalence of infection by Listeria monocytogenes is very low, which is consistent with the reports of other case series. Although the prevalence of herpes simplex infection is low, we need to take into account the considerable morbidity and mortality associated to it in these patients.
Conflict of interestsThe authors have no conflict of interests to declare.
We thank the members of the Working Group on the Febrile Infant of the Research Network of the Sociedad Española de Urgencias de Pediatría (RISeuP-SPERG) for their contributions to this study. The researchers in each hospital were: Andrés González (H. Universitario de Basurto), Anna Fabregas (H. Universitario Vall d’Hebron de Barcelona), Isabel Durán (H. Universitario Carlos Haya de Málaga), Sandra Moya (Corporació Sanitària Parc Taulí de Sabadell), M. Luisa Herreros (H. Universitario Infanta Sofía), Jesús Rodríguez (H. Universitario Virgen de la Arrixaca de Murcia), David Montes (H. Universitario de Fuenlabrada), Fernando Uribarri (H. San Rafael de Madrid), Fernando de la Zerda (H. de Nens de Barcelona), Elisa García (H. de Cabueñes, Gijón), Esther Crespo (H. Universitario Virgen de la Salud de Toledo), Mariano Plana (H. Universitari Arnau de Vilanova de Lleida), Lorena Moreno (H. Universitario Virgen de las Nieves de Granada), Arístides Rivas (H. Universitario Gregorio Marañón de Madrid), Ignacio Manrique (H. Quirón de Valencia), Agustín Rodríguez (H. Alto Deba de Arrasate-Mondragón).
Please cite this article as: de la Torre M, de Lucas N, Velasco R, Gómez B, Mintegi S, Grupo para el estudio del lactante febril de la Red de investigación de la Sociedad Española de Urgencias de Pediatría (RISeuP-SPERG). Etiología y evolución de las infecciones potencialmente graves en lactantes menores de 3 meses febriles. An Pediatr (Barc). 2017;87:42–49.
Previous presentations: this study was presented at the 19 Reunión de la Sociedad Española de Urgencias de Pediatría; April 3–5, 2014; Sabadell, Spain; and the 9th European Congress on Emergency Medicine EUSEM; September 28–October 1, 2014; Amsterdam, the Netherlands.