The inclusion of the 13-valent pneumococcal conjugate vaccine in the routine immunization schedule of the Autonomous Community of Valencia (ACV) started on March 1, 2015 for children born January 1, 2015 and after,1 in accordance with the agreement of the Interterritorial Council of the Spanish Public Health System of January 14, 2015.
Scientific associations such as the Asociación Española de Pediatría (Spanish Association of Paediatrics) had included it with a 2+1 dose schedule when given as part of the routine immunization schedule and a 3+1 schedule if the choice to vaccinate was made on a case-to-case basis.2 A 3+1 schedule was recommended for all children belonging to risk groups.
A document for health care professionals that explained the 2+1 schedule to be implemented3 was drafted in the ACV in February 2015 and reviewed by scientific associations in the field of paediatrics: Sociedad Valenciana de Pediatría (Valencian Association of Paediatrics), Asociación Valenciana de Pediatría en Atención Primaria (Valencian Association of Primary Care Paediatrics), Asociación de Pediatría Extrahospitalaria de Alicante (Association of Outpatient Paediatrics of Alicante) and the Advisory Committee on Vaccines of the ACV.
Despite these recommendations, some paediatricians continue to recommend the 3+1 schedule in all children and not only those in risk groups, because they believe it is necessary to achieve herd immunity.
For this reason, we set out to determine the PCV13 coverage rates and dose schedules used from the time it was included in the routine immunization schedule of the ACV4 in children born between January 1 and September 30, 2015, and assessing potential additional costs for parents and the health care system.
We conducted a cross-sectional descriptive study using data collected from the Vaccine Information System (Sistema de Información Vacunal [SIV])4 of both the Nominal Vaccine Register (Registro de Vacunas Nominal [RVN]) and the Vaccine Repository Register (Registro de Vacunas de Almacén [RVA]), using the Population Information System (Sistema de Información Poblacional [SIP]) or public health card as a population register.
We studied the following variables: health area and basic health zone, age, number of administered doses, vaccine lot number, and risk-group membership. We performed the statistical analysis with Microsoft Excel® 2003.
In the period under study, 31815 children were born, and 42005 PCV13 doses administered, out of which 23670 were first doses, 16096 second doses and 2239 third doses. The mean age-adjusted vaccine coverage rate was 93.6% for the first dose (95% IC, 93.5–93.7), 88.5% for the second dose (95% CI, 88.3–88.6) and 20.4% for the third dose (95% CI, 20.1–20.7). Of the children that received a third dose, 92.6% did not belong to any risk groups. The proportion of children with no risk that received the first dose was 98.3%.
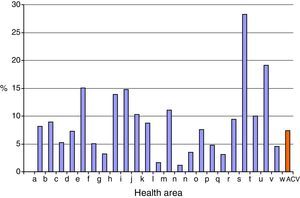
Fig. 1 shows the percentage of children born in 2015 that belonged to risk groups and received a third dose of PCV13 by health area.
Of the 24 health areas of the ACV, four had coverage rates for a third dose of PCV13 vaccine that exceeded 49% (Table 1).
Health areas with the highest coverage rates for the PCV13 vaccine in the ACV, and percentage of children that did not belong to any risk groups.
| Area | Third dose | Children not at risk | |||
|---|---|---|---|---|---|
| N | Coverage (%) | 95% CI | N | % | |
| A | 62 | 68.9 | 58.2–79.6 | 60 | 96.8 |
| B | 169 | 59.7 | 53.7–65.7 | 167 | 98.8 |
| C | 211 | 49.3 | 46.8–51.8 | 195 | 92.4 |
| D | 220 | 55.2 | 52.6–57.7 | 210 | 95.5 |
| Total | 662 | – | – | 632 | 95.9 |
ACV, Autonomous Community of Valencia; CI, confidence interval.
In these health areas, 91.9% of the third doses were not supplied by the public health system, so we assume that these doses were purchased at pharmacies by parents.
The total expenditure in third doses for children that did not belong to any risk group in the ACV amounted to 158176.48€, of which 43304.80€ corresponded to the Valencian Public Health System for vaccination events that were covered under the tax laws5 of the Government of the ACV.
The PCV13 vaccine coverage rate in the ACV in 2014, before the vaccine was included in the routine immunization schedule, was 67%,4 which already sufficed to achieve herd immunity. Therefore, a third dose at age 6 months is not justified by the vaccination coverage rates either before or after the inclusion of the PCV13 vaccine in the routine schedule, save in children belonging to risk groups.
High coverage rates have been achieved since the PCV13 vaccine was first included in the routine immunization schedule, so a third dose at 6 months would not be indicated except in at-risk children. The additional and inefficient costs for both parents and the Department of Public Health are considerable. When it comes to vaccination, more is not always better, so we must apply the best and most efficient scientific evidence that is currently available for the benefit of the population and immunization programmes.
Please cite this article as: Boone ALD, Besó-Delgado M, Alguacil-Ramos AM, Pastor-Villalba E, Portero-Alonso A. Más no siempre es mejor. Pauta individual o calendario sistemático en vacuna antineumocócica. An Pediatr (Barc). 2016;85:320–321.