Acute bronchiolitis (AB) of the infant has a serious outcome in 6–16% of the hospital admitted cases. Its pathogenesis and evolution is related to the response of the T lymphocytes. The objective of the present study is to determine if the lower systemic lymphocytic response is related to a worse outcome of AB in hospitalised infants.
Patients and methodRetrospective observational-analytical study of cases–controls nested in a cohort of patients admitted due to RSV-AB between the period from October 2010 to March 2015. Those with a full blood count in the first 48h of respiratory distress were included. Infants with underlying disease, bacterial superinfection, and premature infants <32 weeks of gestation were excluded. The main dichotomous variable was PICU admission. Other variables were: gender, age, post-menstrual age, gestational and post-natal tobacco exposure, admission month, type of lactation, and days of onset of respiratory distress. Lymphocyte counts were categorised by quartiles. Bivariate analysis was performed with the main variable and then by logistic regression to analyse confounding factors.
ResultsThe study included 252 infants, of whom 6.6% (17) required PICU admission. The difference in mean±SD of lymphocytes for patients admitted to and not admitted to PICU was 4044±1755 and 5035±1786, respectively (Student-t test, P<0.05). An association was found between PICU admission and lymphocyte count <3700/ml (Chi-squared, P=0.019; OR: 3.2) and it was found to be maintained in the logistic regression, regardless of age and all other studied factors (Wald 4.191 P=0.041, OR: 3.8).
ConclusionsA relationship was found between lymphocytosis <3700/ml in the first days of respiratory distress and a worse outcome in previously healthy infants <12 months and gestational age greater than 32 weeks with RSV-AB.
La bronquiolitis aguda (BA) del lactante tiene una evolución grave entre el 6 y el 16% de los casos ingresados. Su patogenia y evolución está relacionada con la respuesta de los linfocitos T. El objetivo del presente estudio es comprobar si la menor respuesta linfocitaria sistémica está relacionada con una peor evolución de la BA en lactantes ingresados.
Pacientes y métodoEstudio observacional-analítico retrospectivo de casos-controles anidados en una cohorte de ingresados por BA-VRS en el periodo de octubre del 2010 a marzo del 2015. Se incluyó a aquellos con hemograma en las primeras 48h de dificultad respiratoria. Se excluyó a los lactantes con patología de base, sobreinfección bacteriana y prematuros ≤ 32 semanas de gestación. La variable principal dicotómica fue ingreso UCIP. Otras variables fueron: sexo, edad, edad posmenstrual, exposición gestacional y posnatal al tabaco, mes de ingreso, tipo de lactancia y días de evolución del distrés respiratorio. Las cifras de linfocitos fueron categorizadas por cuartiles. Se realizó un análisis bivariante con la variable principal y posteriormente regresión logística para analizar factores de confusión.
ResultadosEl estudio incluyó a 252 lactantes. El 6,6% (17) precisó UCIP. La diferencia de media±DE de linfocitos para pacientes ingresados y no ingresados en UCIP fue de 4.044±1.755 y 5.035±1.786, respectivamente (t de Student, p<0,05). Se encontró asociación entre ingreso UCIP y la cifra de linfocitos < 3.700/ml (Chicuadrado p=0,019; OR: 3,2), que se mantuvo en la regresión logística con independencia de la edad y del resto de factores estudiados (Wald 4,191 p=0,041; OR: 3,8).
ConclusionesExiste relación entre la linfocitosis < 3.700/ml en los primeros días de la dificultad respiratoria y una peor evolución en lactantes < 12 meses previamente sanos y edad gestacional mayor de 32 semanas con BA-VRS.
Acute bronchiolitis (AB) has a more severe course in infants, with 6–16% of those admitted to hospital requiring supportive care in paediatric intensive care units (PICUs).1–3 Various studies have sought to identify factors associated with a severe course of disease,2,4–8 but when it comes to previously healthy children with no predisposing factors, the current evidence has not clearly established which infants may be at higher risk.9 There is widespread agreement that preterm and young infants are most likely to require higher levels of care2 (and that respiratory syncytial virus (RSV) is the most frequent and virulent aetiological agent involved in cases in infants.5,10,11 There is also evidence of an association between the month of birth and the severity of AB.12,13 It is unclear whether the presence of viral coinfection increases the severity of disease, although there is a growing consensus that bacterial superinfection increases the length of stay and the need for supportive care.2,14,15
Infants are at high risk of severe complications of viral respiratory tract infections. It is known that the pathogenesis of AB is associated with the activity of T-helper (Th) lymphocytes, which are recruited at the level of the respiratory epithelium by different cytokines and chemokines secreted in response to infection, and specifically with an increased CD8+/CD4+ T cell ratio, which is higher the more severe the disease.16 In this regard, some authors have suggested the possibility that systemic lymphocyte counts, which are highly correlated with the increase in the levels of interferon gamma (INFγ) in the acute phase of infection, may be associated with a more severe course of AB, reflecting a skewing towards a Th2-dominated response of an allergic/inflammatory nature.17,18
The aim of our study was to determine whether the systemic lymphocyte response in RSV infection was associated with poorer outcomes in a series of infants admitted with AB in the absence of comorbidity.
Patients and methodsWe conducted an observational and analytical case–control study in a cohort of children admitted with a diagnosis of AB between October 1, 2010 and March 31, 2015. We were able to conduct this study because AB is a frequent disease whose management is standardised, so that the electronic health records of our hospital contained data on the main variables under study that were actively documented per protocol in the nursing and medical notes.
We considered patients aged less than 12 months that met the classical criteria for diagnosis of AB19 and with detection of RSV antigen in nasopharyngeal aspirate samples using previously described methods20 during the 5 epidemic seasons under study. We reviewed the charts of every previously healthy infant with AB by RSV for every day they spent in hospital between admission and discharge and selected the patients whose symptoms had led to performance of a complete blood count within 48h from the onset of respiratory distress. We excluded patients with underlying disease, previously treated with glucocorticoids or palivizumab or born preterm before 32 weeks’ gestation, as there is evidence that infants of these characteristics are likely to develop more severe forms of disease or to have been treated with palivizumab.21 We also excluded patients whose test results confirmed or suggested the presence of bacterial superinfection, which included patients with positive blood or urine culture, presence of white blood cells and/or nitrites in urine or elevation of acute phase reactants (such as C-reactive protein >70mg/L and/or procalcitonin >0.5ng/mL), or in whom the physician suspected bacterial superinfection regardless of the results of diagnostic tests based on previously published criteria.14,15
The primary outcome variable was the need for PICU admission during the hospital stay due to the need for mechanical ventilation, including any invasive modality (conventional or high frequency mechanical ventilation) or non-invasive modality (non-invasive continuous positive pressure, including CPAP or BiPAP) and excluding high-flow nasal cannula oxygen therapy. We expressed this endpoint as a dichotomous variable: yes/no. Table 1 shows the criteria generally applied for admission to the PICU.
Also, for every patient, we collected data recorded at admission on variables that could behave as confounders: sex, age, postmenstrual age, exposure to tobacco during gestation, month of admission, type of infant feeding, presence of apnoea and days elapsed from onset of respiratory distress. We also collected the level of haemoglobin level and the neutrophil, lymphocyte, eosinophil, basophil and monocyte counts, and categorised them based on the distribution quartiles for the purpose of statistical analysis. We created two categories for the postmenstrual age variable based on whether the infant had reach the age of 44 weeks, considered the lower bound of the neonatal period independently of gestational age at birth.
After the retrospective collection of followup data for the cohort, we classified infants that required the supportive care included in the definition of the primary outcome during their stay as cases, and all other infants as controls. We performed the statistical analysis with the GNU PSPP freeware. We have summarised qualitative variables as percentages with the corresponding confidence intervals (CIs) for the primary outcome value, and quantitative variables as mean and standard deviation.
To assess potentially associated factors, we compared PICU admission with other quantitative variables using the Student t test, and performed bivariate analyses of qualitative data by means of the chi square or the Fisher exact test, as applicable, comparing the primary outcome variable (PICU admission) to the lymphocyte count and the other independent variables under study. To control confounding, we fitted a multivariate binomial logistic regression model for the association of the primary outcome variable and the lymphocyte count as well as the remaining factors, including all variables for which the P-value had been less than 0.20 in the bivariate analysis as well as clinical variables identified as relevant in previous studies in the initial model. We defined statistical significance as a P-value of less than 0.05 in any of the tests, and always calculated confidence intervals with a width of 95%.
The study was approved by the ethics committee of our hospital and exempted from the need of obtaining informed consent, as it was a retrospective observational study of anonymised data.
ResultsIn our sample of 1197 patients admitted with bronchiolitis during the period under study, RSV was detected in the nasopharyngeal aspirate samples of 901. We found that blood tests had been ordered in 434 of these RSV-positive patients in the first 48h following onset of respiratory distress to assess the symptoms. Of these patients, 127 received a diagnosis of severe bacterial infection in association with AB based on the previously noted criteria, and were therefore excluded from the study. We also excluded infants with underlying disease, patients older than 12 months and infants born preterm before 32 weeks’ gestation. The final sample included 252 infants with BA due to RSV that were previously healthy and with no evidence of bacterial infection. Table 2 summarises the demographic characteristics of the sample.
Descriptive analysis of previously healthy infants aged less than 12 months with bronchiolitis caused by RSV.
| N | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| Birth weight (g) | 243 | 1440 | 5000 | 3162 | 565 |
| Gestational age (weeks) | 252 | 33.0 | 42.0 | 38.41 | 1.83 |
| Age (months) | 252 | 0.16 | 10.12 | 2.42 | 1.64 |
| Postmenstrual age (weeks) | 247 | 36.14 | 85.00 | 48.87 | 7.35 |
| Maternal age (years) | 187 | 15 | 44 | 29.32 | 6.24 |
| Length of hospital stay (days) | 252 | 1 | 25 | 6.34 | 3.30 |
| Haemoglobin (g/dL) | 252 | 8.2 | 17.3 | 11.25 | 1.69 |
| Neutrophils/mL | 252 | 900 | 17,989 | 5497 | 3141 |
| Monocytes/mL | 252 | 280 | 19,430 | 6969 | 2749 |
SD, standard deviation.
In our sample, 17 patients were admitted to the PICU during their hospital stay, corresponding to an incidence of 6.6% (95% CI, 4–10%). Table 3 presents the results of the quantitative analysis, showing statistically significant differences in the lymphocyte count and postmenstrual age based on PICU admission. Table 4 shows the frequency distributions of the qualitative variables, overall and by PICU admission, with the corresponding P-value obtained in the bivariate analysis. The key findings shown in Table 4 are the association between PICU admission and the lymphocyte count category, postmenstrual age of less than 44 weeks, presence of apnoea and fever. We did not find an association between age group and the distribution of lymphocytes by quartiles (Table 5).
Student t test for comparison of quantitative variables in infants with acute bronchiolitis.
| PICU (yes=1, no=0) | N | Mean | Standard deviation | P (t-test) | |
|---|---|---|---|---|---|
| Haemoglobin (g/dL) | 0 | 235 | 11.39 | 1.92 | 0.637 |
| 1 | 17 | 11.62 | 2.08 | ||
| Leucocytes (cells/mL) | 0 | 235 | 12,576 | 4314 | 0.083 |
| 1 | 17 | 10,691 | 4182 | ||
| Neutrophils (cells/mL) | 0 | 234 | 5652 | 3141 | 0.357 |
| 1 | 17 | 4932 | 2570 | ||
| Lymphocytes (cells/mL) | 0 | 232 | 5035 | 1786 | 0.028 |
| 1 | 17 | 4044 | 1765 | ||
| Eosinophils (cells/mL) | 0 | 229 | 116 | 148 | 0.319 |
| 1 | 17 | 80 | 63 | ||
| Monocytes (cells/mL) | 0 | 229 | 1142 | 484 | 0.896 |
| 1 | 17 | 1158 | 488 | ||
| Basophils (cells/mL) | 0 | 228 | 134 | 117 | 0.206 |
| 1 | 17 | 98 | 61 | ||
| Platelets (cells/mL) | 0 | 231 | 475,601 | 159,151 | 0.320 |
| 1 | 17 | 435,764 | 160,240 | ||
| Birth weight (g) | 0 | 228 | 3167 | 574 | 0.647 |
| 1 | 15 | 3098 | 397 | ||
| Weeks of gestation | 0 | 241 | 38.42 | 1.85 | 0.770 |
| 1 | 17 | 38.28 | 1.68 | ||
| Postmenstrual age (weeks) | 0 | 232 | 49.19 | 7.42 | 0.001 |
| 1 | 15 | 43.84 | 3.45 | ||
Frequency distribution of qualitative variables in patients aged less than 12 months with no underlying disease and born at more than 32 weeks’ gestation admitted to hospital with acute bronchiolitis due to RSV.
| Total sample, % (N=252) | PICU, % (n=17) | No PICU, % (n=235) | P (χ2 test) | |
|---|---|---|---|---|
| Male sex | 50.4 | 52.9 | 50.2 | 0.828 |
| Weight <3rd %ile | 4.5 | 6.4 | 4.6 | 0.646 |
| Gestational age | 0.859 | |||
| >37 weeks | 86.8 | 88.2 | 86.7 | |
| 32–36 weeks | 13.2 | 11.8 | 13.3 | |
| Birth during high-risk season | 36.8 | 64.7 | 34.9 | 0.019 |
| Distress lasting <48h | 77.0 | 81.3 | 76.7 | 0.911 |
| Lymphocytes (first quartile), cells/mL | 0.034 (FET) | |||
| <3700 | 23.7 | 47.1 | 22.0 | |
| ≥3700 | 24.8 | 11.8 | 25.7 | |
| Breastfeeding | 43.5 | 50.0 | 43.0 | 0.585 |
| Postmenstrual age <44 weeks | 27.5 | 66.7 | 25.0 | <0.001 |
| Maternal smoking during pregnancy | 14.6 | 5.9 | 15.2 | 0.292 |
| Environmental exposure to smoke | 33.5 | 29.4 | 38.8 | 0.713 |
| Fever >38°C | 53.9 | 25.0 | 55.8 | 0.017 |
| Episodes of apnoea | 8.2 | 35.3 | 6.3 | <0.001 |
FET, Fisher exact test; PICU, paediatric intensive care unit; RSV, respiratory syncytial virus; %ile, percentile.
Distribution by quartiles of lymphocytes by age group in infants with acute bronchiolitis due to RSV.
| Lymphocytes (distribution by quartiles) | Total | ||||||
|---|---|---|---|---|---|---|---|
| ≤3700.00 | 3701.00–4900.00 | 4901.00–6180.00 | 6181.00+ | ||||
| Age groups | <1 month | Count | 14 | 11 | 9 | 10 | 44 |
| % | 31.8 | 25.0 | 20.5 | 22.7 | 100.0 | ||
| 1–3 months | Count | 33 | 32 | 36 | 29 | 130 | |
| % | 25.4 | 24.6 | 27.7 | 22.3 | 100.0 | ||
| >3 months | Count | 16 | 19 | 17 | 23 | 75 | |
| % | 21.3 | 25.3 | 22.7 | 30.7 | 100.0 | ||
| Total | Count | 63 | 62 | 62 | 62 | 249 | |
| % | 25.3% | 24.9 | 24.9 | 24.9 | 100.0 | ||
Chi square, n=258; P=0.736.
Table 6 presents the results of the multivariate binomial logistic regression analysis of the need of supportive care in the PICU based on the independent variables included in the model. The statistically significant factors identified in the analysis were postmenstrual age of less than 44 weeks and a lymphocyte count below the first quartile, which in our cohort of infants corresponded to a count of less than 3700lymphocytes/mL.
Binomial logistic regression analysis of the need for supportive care at the PICU level in the sample of infants with RSV bronchiolitis.
| B | Standard error | Wald | Sig. | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Lymphocytes <3.700/ml | 1.332 | 0.651 | 4.191 | 0.041 | 3.790 | 1.058 | 13.571 |
| Fever <38°C | 0.666 | 0.677 | 0.967 | 0.325 | 1.946 | 0.516 | 7.338 |
| High-risk birth | 1.977 | 0.677 | 8.528 | 0.003 | 7.224 | 1.916 | 27.234 |
| Postmenstrual age <44 weeks | 1.384 | 0.660 | 4.398 | 0.036 | 3.991 | 1.095 | 14.547 |
| Apnoea before PICU | 1.792 | 0.973 | 3.393 | 0.065 | 6.001 | 0.892 | 40.395 |
The findings of our study suggest that a decreased systemic lymphocyte response is associated with increased severity of disease. Therefore, the former could be considered a risk factor for poor outcome, independently of the month of birth, fever, age, postmenstrual age of less than 44 weeks or the presence of apnoea (Table 6). The bivariate analysis also identified exposure to smoke, duration of respiratory distress and the type of milk fed to the infant as independent risk factors (Table 4).
Acute bronchiolitis by RSV involves primary infection of a host that has not developed immunity against this virus, and the pathogenesis and course of the disease are different from those of episodes of reinfection that occur later in life.9 The risk of AB becoming severe depends on the virulence of the causative agent and the individual susceptibility of the patient, which depends on the maturity and performance of the immune system.
As for virulence, it appears that RSV is the most prevalent agent involved in respiratory infections in infants and is also associated with a higher severity of disease,5,10,11 as reflected by the total length of stay, need for respiratory support and need for overall care.22 In our study, the 6.6% incidence of PICU admission refers to infection in healthy infants without bacterial infection or other risk factors. Thus, we report the incidence of PICU admission attributable to bronchiolitis alone, which explains why this figure is lower than the overall incidence reported by previous studies.1,2,23,24
In infants, the immaturity of the immune system entails a predominance of thymus-derived nonspecific T cells in circulating immune cells compared to adults. The regulation and production of memory T cells occurs in the intestinal and bronchial epithelia.25 The pathogenesis of AB involves the balance of Th1/Th2 response cytokines and its cytopathic effect. The main cytokine involved in the Th1 response is IFN-γ which, leading the innate immune response, limits viral replication and counteracts the activity of Th2 cells, which is directly associated with the cytokine interleukin 4 (IL-4). There is evidence that a skewing towards a Th2 response is associated with greater severity of AB26 and a lower lymphocyte count, as we also found in our study. The most appropriate way to assess such a shift is to use ratios for molecules involved in either pathway, such as the IL-4/IFN-γ ratio. There is evidence that absence of IFN-αβ and IFN-γ signalling after viral infection is associated with an insufficient T cell response, a response that under normal circumstances accounts for the majority of circulating lymphocytes, resulting in decreased viral clearance, which would explain the increased severity of AB.27
Furthermore, other authors have found reduced levels of IFN-γ and lower T cell counts in infants with moderate to severe AB, which they attributed to a potential inhibitory effect of the virus on the innate response that would compound the immaturity of the infant's immune system.17 This effect would in fact be characteristic of infection by RSV compared to infection by other pathogens in infants.28 Primary infection by RSV is followed by mechanisms of proliferation and apoptosis that modulate the immune response that will be reflected in the complete blood count during the acute phase of infection.29 A recent study has demonstrated that RSV suppresses the Th1 response through partial inhibition of the IFN response, in the acute phase and for months following infection, a phenomenon that has been labelled “immune dysregulation” by RSV.30,31 There is evidence that maturation of the infant with increasing age protects against infection.32,33
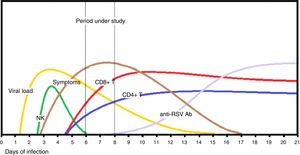
The main strength of our study was that we were able to retrospectively restrict the cohort of cases to infants with AB and without associated complications that could contribute to disease severity, with a careful selection of previously healthy infants and a narrow range in the duration of symptoms prior to admission, which usually corresponded to the arrival to hospital of the infant at the onset of respiratory distress, when the disease was clearly established and in the acute phase, as can be seen in Fig. 1. The established threshold of 3700lymphocytes/mL may seem arbitrary, but it is useful and was chosen based on the analysis of the distribution of cell counts in the sample. It corresponds to the first quartile in the infants admitted with BA that fulfilled the inclusion criteria. Although there are previous reports of an impaired lymphocyte response in severe cases of AB,17,18 we offer this value as a tentative threshold that may be useful in clinical practice. One of the conclusions that may be drawn from our findings is that performance of a complete blood count in selected cases of AB may be useful in assessing the likelihood of a severe course of disease.
Sequence of events in the immune response to primary infection by RSV in infants with acute bronchiolitis. After the prodromal incubation period, the viral load peaks at around day 4, coinciding with the activation of natural killer (NK) cells, which release interferon-γ, and followed by CD4+ and CD8+ T cell recruitment. Upper respiratory symptoms appear between days 3 and 5 from infection, and respiratory distress develops about 48h after the onset of respiratory symptoms. The production of surface protein neutralising antibodies (anti-RSV Abs) occurs at a later stage, as the virus is cleared and symptoms improve.
Among the limitations of our study, we ought to highlight that we did not analyse the highly probable presence of viral coinfection, with has been found in up to 30% of patients of AB in case series conducted in neighbouring regions.34 We also did not have data to differentiate between RSV serotypes A and B. Furthermore, the sample was small and we obtained wide confidence intervals, so prospective multicentre studies would be needed to corroborate our findings.
In conclusion, our study found an association between a circulating lymphocyte count of less than 3700cells/mL in the first 48h of respiratory distress and increased severity of disease in previously healthy infants aged less than 12 months and born after 32 weeks’ gestation with AB due to RSV.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ramos-Fernández JM, Moreno-Pérez D, Antúnez-Fernández C, Milano-Manso G, Cordón-Martínez AM, Urda-Cardona A. Menor respuesta linfocitaria en casos graves de bronquiolitis aguda por virus respiratorio sincitial. An Pediatr (Barc). 2018;88:315–321.