Late preterm (LP) infants (34–36 weeks of gestation) are the largest group of preterm infants and also the least studied so far. In order to improve their care and reduce the impact of their increased morbidity and mortality, it is essential to know the current situation in Spain.
Population and methodClinical-epidemiological variables of the LP population of 34 participating hospitals were prospectively collected from April 1, 2011 to March 31, 2016, and were then compared with the Minimum Perinatal Data Set for term births in the database.
ResultsOf the 9,121 LP studied, 21.7% of 34, 30.8% of 35, and 47.5% of 36 weeks of gestation. The mortality rate was 2.8%. More than one-quarter (27.7%) were multiple pregnancies. Maternal diseases were identified in 47.1% and 41.4% were pathological gestation. Just under half (47.9%) were by Caesarean section and 18.8% were of unknown origin or unjustified. No known cause of prematurity was found in 29%, and 3.1% were recognised as unjustified. Just under half (47%) of the LP were breastfed, and 58.6% required admission to neonatology, with 15.2% to Neonatal Intensive Care Unit. Coded diagnoses were recorded in 46.2%, with the most frequent being jaundice, 43.5%, hypoglycaemia, 30%, and respiratory disorders with 28.7%.
ConclusionsThe large sample of LP studied helps us to highlight the higher neonatal mortality and morbidity that this population suffers and the unavoidable relationship of its incidence with multiparity, maternal ageing, and the still numerous inductions of labour and unjustified elective caesareans.
Los prematuros tardíos (PT) (34-36 semanas de gestación) son el grupo más amplio de prematuros y menos estudiado hasta ahora. Para mejorar sus cuidados y disminuir el impacto de su mayor morbimortalidad, es primordial conocer su realidad en nuestro país.
Población y métodoSe recogen prospectivamente variables clínico-epidemiológicas de la población de PT de 34 hospitales participantes, desde el 1 de abril del 2011 al 31 de marzo del 2016. Se comparan con las de la base de datos Conjunto Mínimo de Datos Perinatales para nacidos a término.
ResultadosSe estudia a 9.121 PT, el 21,7% de 34, el 30,8% de 35 y el 47,5% de 36 semanas de gestación. Falleció el 2,8‰. El 27,7% fueron embarazos múltiples, el 47,1% identificó enfermedades maternas y el 41,4% patología gestacional. Nacieron por cesárea el 47,9%, el 18,8% de origen no conocido o injustificado. En un 29% no se encontró causa conocida de prematuridad y el 3,1% se reconoció como injustificada. Lactancia materna en el 47%. El 58,6% precisó ingreso en neonatología, el 15,2% en UCIN. El 46,2% codificó algún diagnóstico, los más frecuentes: ictericia (43,5%), hipoglucemia (30%) y trastornos respiratorios (28,7%).
ConclusionesLa numerosa muestra de PT estudiada nos ayuda a poner en relieve la mayor morbimortalidad neonatal que presenta esta población y la ineludible relación de su incidencia con la multiparidad, el envejecimiento materno y las aún numerosas inducciones de parto y cesáreas electivas no justificadas.
According to the report of the Born Too Soon preterm prevention analysis group published in 2013,1 “every year, 1.1 million babies die from prematurity, and many survivors are disabled. Worldwide, 15 million babies are born preterm (<37 weeks’ gestation). The understanding of drivers and potential benefit of preventive interventions for preterm births is poor. We examined trends and estimate the potential reduction in preterm births for countries with very high human development index (VHHDI) if present evidence-based interventions were widely implemented.” In essence, this is the main objective for which the SEN34-36/ACUNA working group of the Sociedad Española de Neonatología (Spanish Society of Neonatology [SENeo]) was created.
Late preterm (LPT) births, that is, births between 34 and 36 weeks’ gestation, account almost entirely for the increase in the rate of preterm birth observed in recent years. In 2005, during an international workshop, a consensus panel acknowledged the vulnerability of LPT infants and decided to discontinue the use of the former phrase “near-term”, which could lead to an underestimation of the actual risk in this population.2,3 There is ample evidence in the literature of the greater morbidity and mortality in LPT infants compared to full term (FT) infants,4–6 and of the impact of LPT birth on psychomotor development.7–9
The SEN34-36 group was constituted in September 2011 with the aim of gaining knowledge on the situation of this population in Spain, for which the SENeo established a register of basic data on perinatal variables, neonatal morbidity and mortality and follow-up through age 2 years using the software Neosoft© 2013 (Hospital La Fe Valencia, Hospital Clínic de Barcelona, Sociedad Española de Neonatología, Abbott Laboratories, Alce Ingeniería) as well as the Proyecto Acuna platform [www.proyectoacuna.es], which is also useful for the followup of these patients, as it provides a standardised method for continuous assessment. The primary objective of the SEN34-36 group is to improve the care provided to LPT infants and their families to decrease neonatal mortality and morbidity and associated sequelae to the extent possible. Among its specific objectives are to establish the incidence of LPT birth, identify the most frequent causes of preterm birth and inpatient morbidity and mortality, and to develop a standardised approach for the followup of these infants in order to identify the sequelae of LPT birth and promote preventive strategies.
The aim of the study was to determine the incidence of late preterm birth and analyse the perinatal data of the broad sample of such births registered by the 58 Spanish hospitals linked to the database as of March 2017 in order to try to bring the world of LPT, which has been underestimated for decades, closer to the collective of obstetricians, neonatologists and primary care paediatricians.
Population and methodWe present the data collected from the specific LPT database to describe the clinical and epidemiological patterns in this population. We collected data for patients that met the gestational age criteria (34+0 to 36+6 weeks, both included) in 34 of the participating hospitals (those that registered every LPT infant delivered in their facilities). We excluded patients delivered in hospitals that registered fewer than 5 LPT births, that only registered LPT infants that required admission to the neonatal ward and/or NICU, or that only registered LPT infants without perinatal disease. The period under study was April 1, 2011 through March 31, 2016. We asked member hospitals for the total number of live births, PT births and PT births before 34 weeks’ gestational age (wGA) managed each year at their facilities. We compared the data we collected for some of the variables with the data documented in the SENeo Minimal Perinatal Dataset (MPNDS) database for children delivered between 37 and 41 weeks of gestation between January 1, 2000 and December 31, 2016.
Statistical methods: We performed a descriptive analysis of the variables under study. This was followed by a bivariate analysis by gestational age, comparing qualitative data by means of the chi square or the Pearson test, and quantitative data by means of analysis of variance or, where the assumption of homogeneity of variance was not met, the Kruskal–Wallis H test. To assess differences between our data and the data collected from the MPNDS database, we used z tests for qualitative data and the Student t test for quantitative data. We defined statistical significance as a p-value of less than 0.05 in any of the tests. The analyses were performed with the statistical software packages SPSS Statistics version 20 (IBM) and R (https://www.r-project.org/).
ResultsBased on all the epidemiologic data received from the 44 affiliated Spanish hospitals, the incidence of preterm birth was 8.3% and the incidence of late preterm birth was 5.9% (71.8% of all preterm births).
The sample under study consisted of 9,121 LPT infants delivered in 34 hospitals selected from the total number of hospitals on the basis that they entered data for every LPT infant born in their facilities. In this sample, 21.7% of infants were born at 34 weeks of gestation, 30.8% at 25 weeks and 47.5% at 36 weeks.
The mean maternal age at the time of delivery was 33 years (SD, 6 years), with 30.8% of mothers aged more than 35 years and 1.8% less than 20 years. In 47.1% of the sample, mothers had a known underlying disease at the time of delivery: endocrine (1.9%), cardiovascular (0.6%), autoimmune (0.5%), respiratory (0.41%) and neurologic (0.21%). The mothers of 9.5% of the infants smoked during gestation, and social problems were identified in 0.9% of the pregnancies.
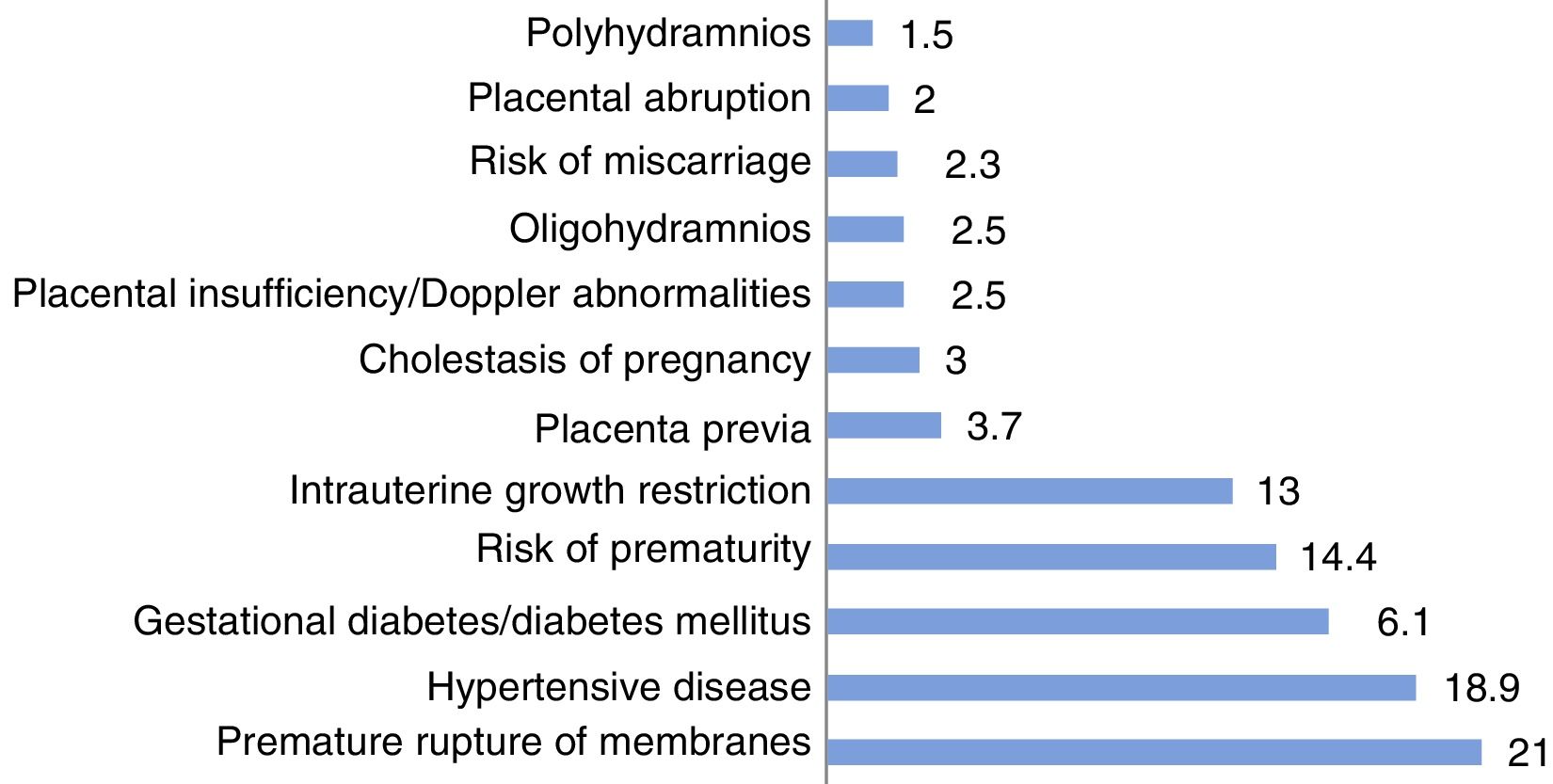
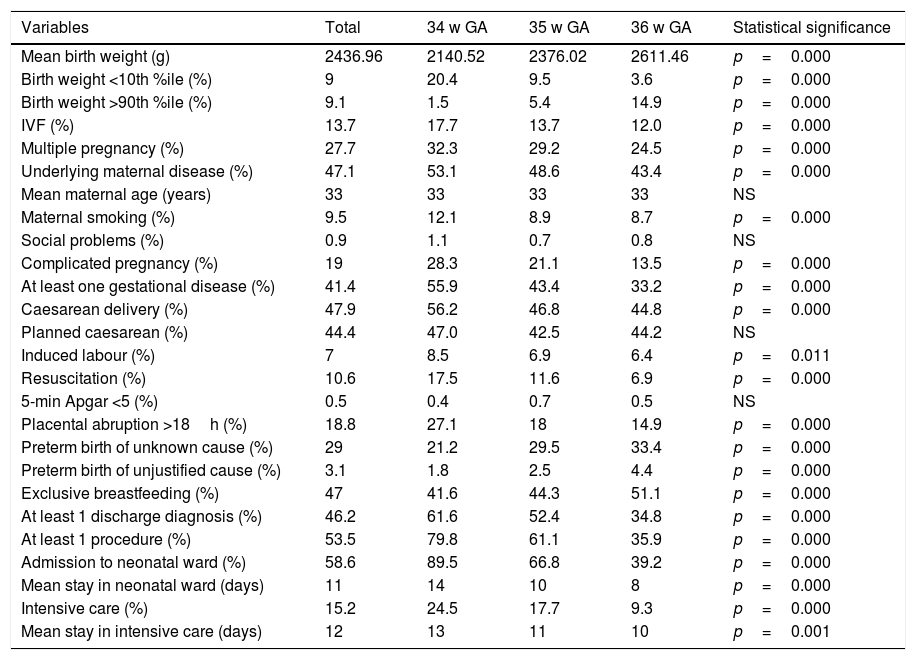
Of all infants, 13.7% were the product of a pregnancy achieved through in vitro fertilisation (IVF) and 27.7% of a multiple pregnancy. The pregnancy was identified as complicated in 19% of the cases and mothers received a diagnosis related to pregnancy complications in 41.4% of cases. The most frequent problems were premature rupture of membranes, hypertensive diseases (preeclampsia/HELLP syndrome, underling high blood pressure), diabetes, risk of premature labour and intrauterine growth restriction (IUGR) (Fig. 1).
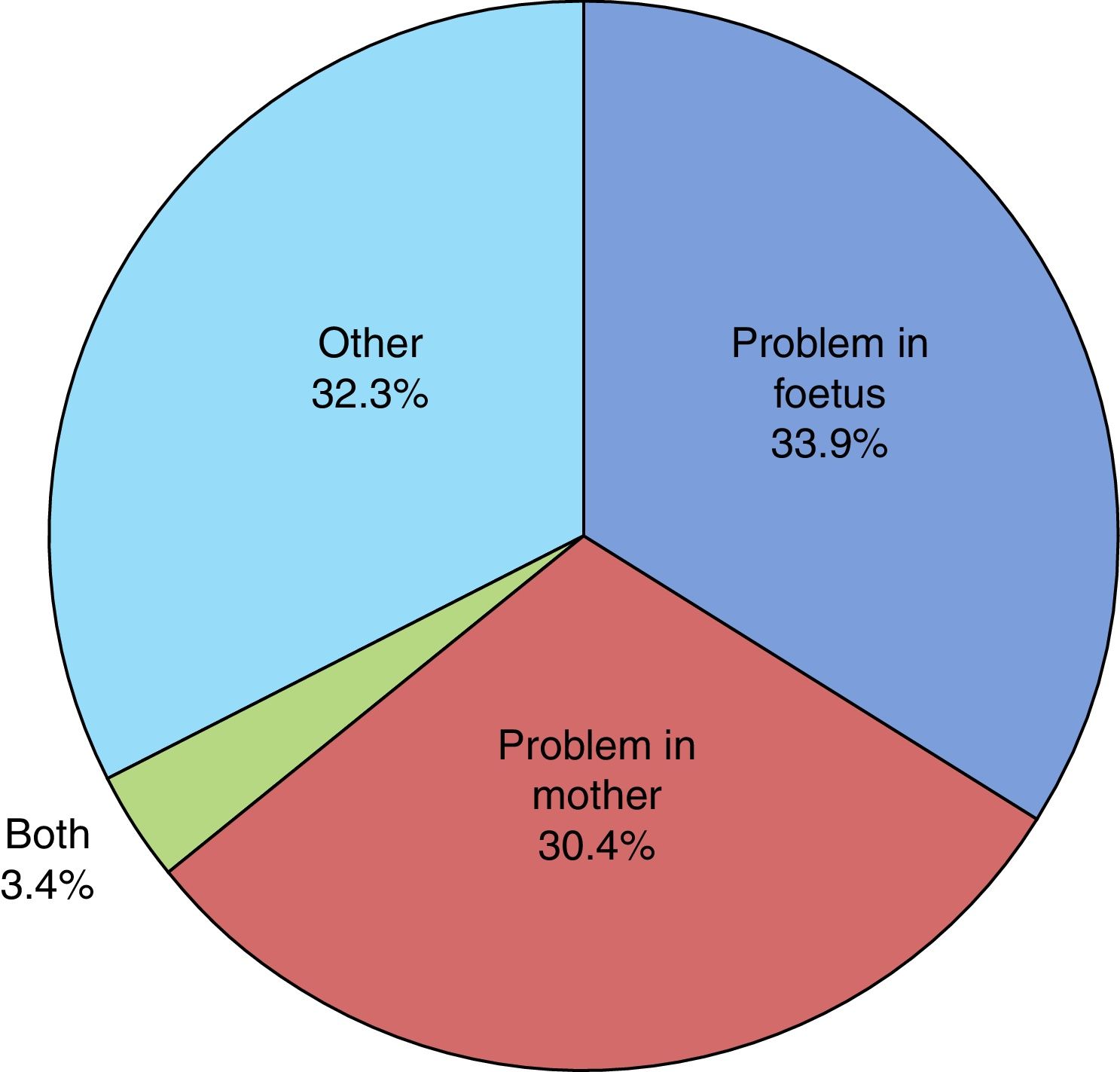
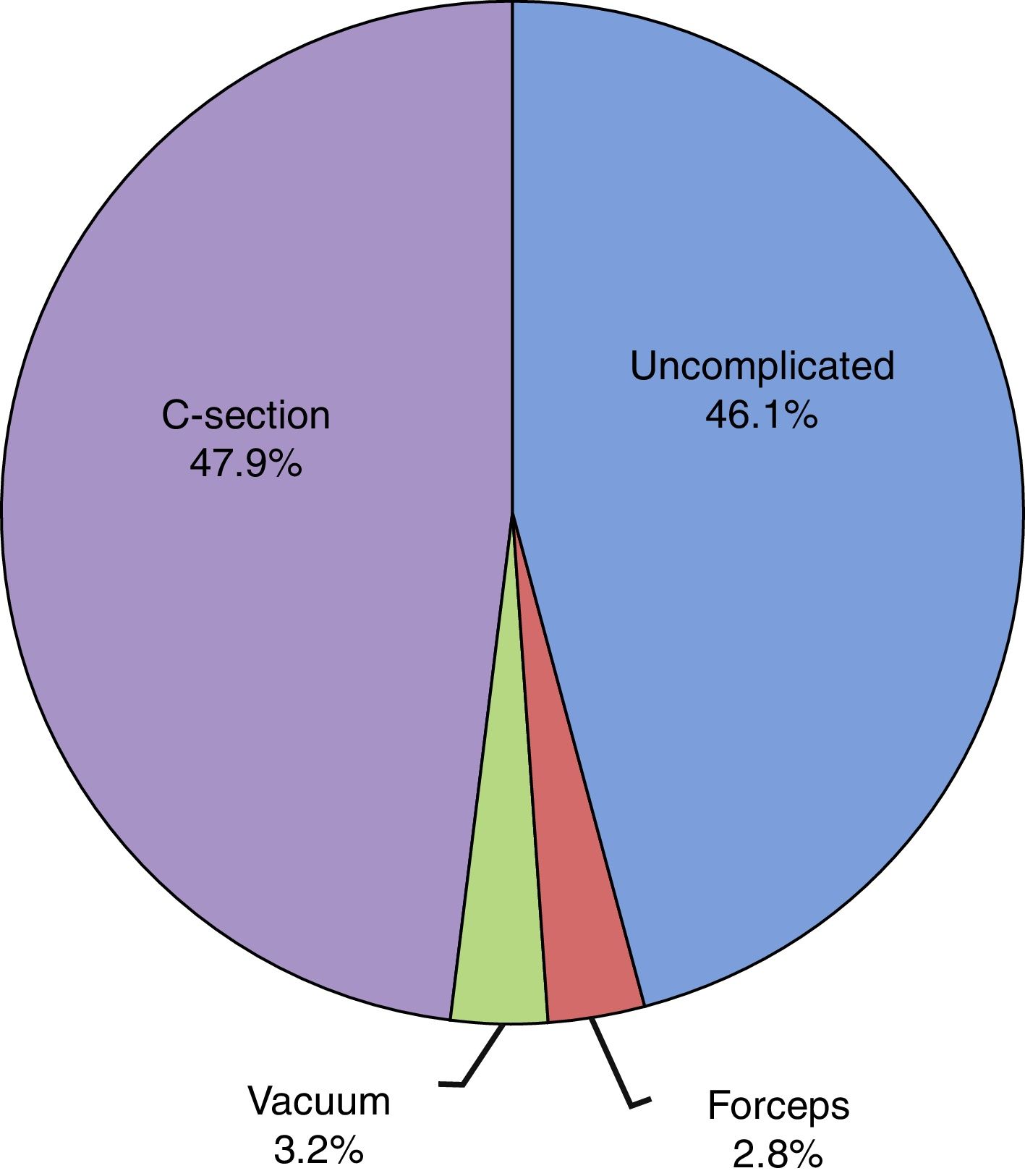
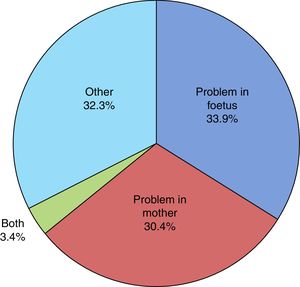
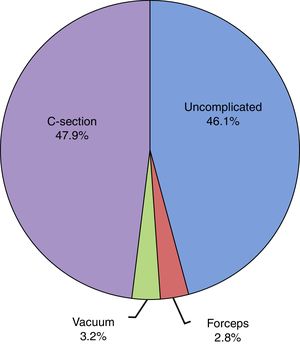
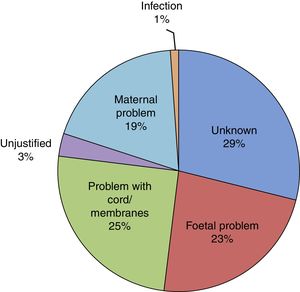
The mean duration of ruptured membranes before delivery was 19.25h (SD, 91.21h), with prolonged premature rupture of membranes of more than 18h in 18.8%. Labour was induced in 7% of cases (Fig. 2). The birth was by caesarean delivery in 47.9% of the sample (Fig. 3), which was planned in 44.4%. The reason for caesarean delivery was a problem in the foetus in 29% of cases, a problem in the mother in 18%, failed induction in 4.3% and labour not progressing in 5.2%, while in 18.8% of cases the reason was not known or the procedure was not justified.
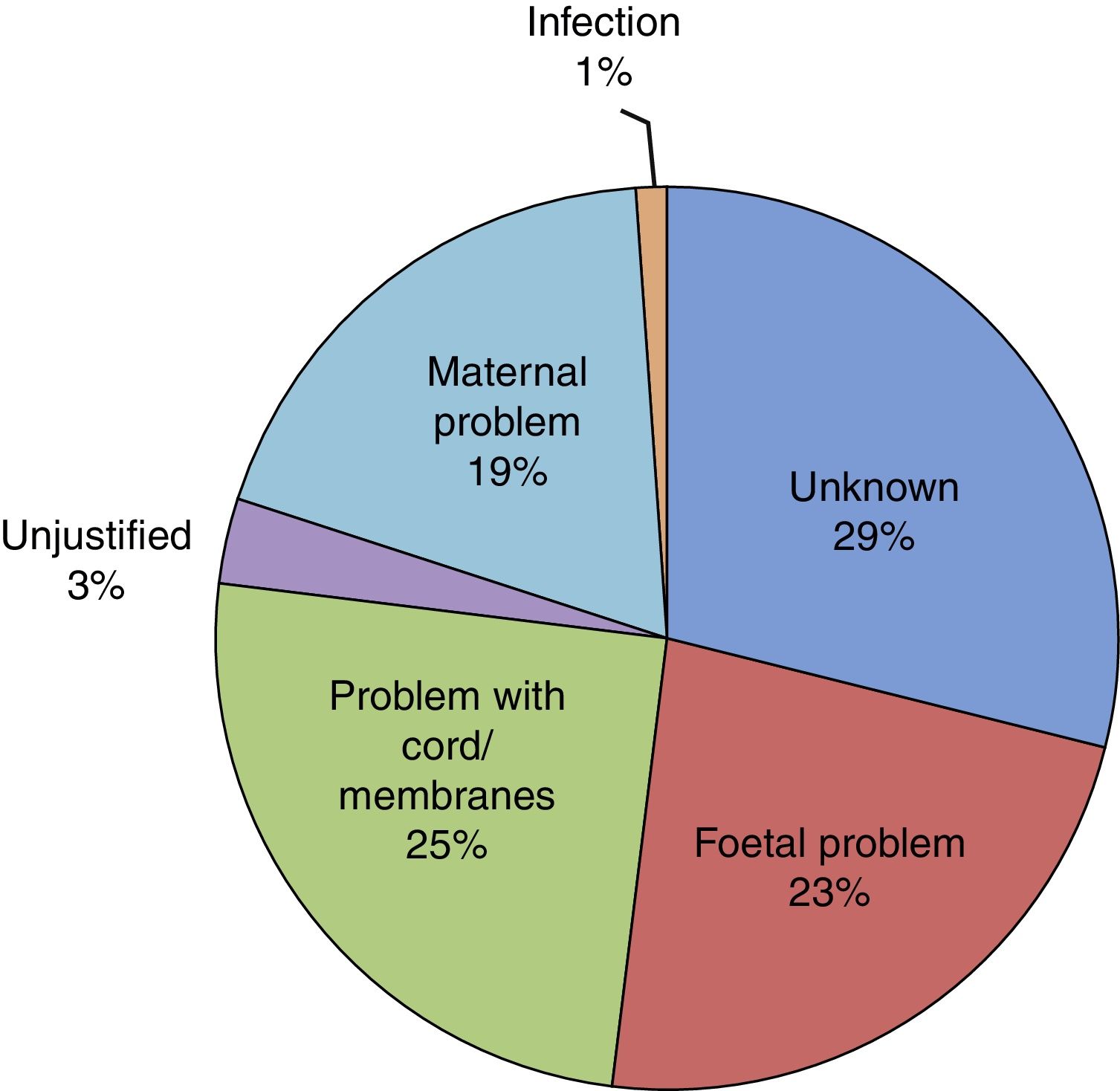
Of all infants, 53.6% were male and 46.4% female. Resuscitation was required at birth in 10.6%, and 0.5% had a 5-min Apgar score of less than 5. The mean birth weight (BW) was 2436.96g (SD, 451.78g), with the BW below the 10th percentile in 19% of the newborns and above the 90th percentile in 9.1%. Twenty-six infants died (2.8‰ of all LPT births). When it came to the distribution by cause of prematurity (Fig. 4), we found that it was unknown in 29% of cases and unjustified in 3.1%. In our sample, 58.6% of infants required admission to the neonatal ward, of who 95.2% stayed for more than 24h. The median length of stay in the ward was 8 days (interquartile range, 5–14 days) and the mean length of stay was 11 days (SD, 11 days). In addition, 15.2%, required admission to the Neonatal Intensive Care Unit (NICU) with a mean length of stay of 12 days (SD, 15 days). At discharge, the rate of exclusive breastfeeding (BF) in LPT infants was 47%, and the rate of mixed BF was 33.9%. A discharge diagnosis code was entered in 46.2% of cases, and the most frequent diagnoses were: jaundice (43.5%), hypoglycaemia (30%), respiratory disorders, including infant respiratory distress syndrome, immature lung, transient tachypnoea of the newborn, pneumothorax and pneumomediastinum (28.7%), low birth weight or length for gestational age (10.8%), difficulty feeding (6.4%), sepsis (3.8%), apnoea (1.6%), suspected infection (0.9%) and persistent pulmonary hypertension of the newborn (0.5%). Hypoglycaemia was one of the discharge diagnoses in 30.1% of newborns delivered at 34 weeks of gestation, 29.9% of those delivered at 35 weeks and 30.0% of those delivered at 36 weeks.
Procedures were performed in 53.5% of LPT infants, the most frequent of which were: phototherapy (27%), intravenous infusion (26.8%), mechanical ventilation (19.1%: continuous positive airway pressure in 14.3% and invasive mechanical ventilation in 4.8%), antibiotherapy (15.9%), tube feeding (11.6%), parenteral nutrition (10%), oxygen therapy (7.9%) and placement of an umbilical catheter in 5.7%. The diagnostic procedures included venepuncture (62.4%), X-ray examination (25.7%) and ultrasound examination (39.1%).
When we analysed the data by gestational age, we found statistically significant differences, with decreasing gestational age associated with increasing proportions of BW below the 10th percentile, IVF, multiple pregnancy, maternal disease prior to delivery, maternal smoking during pregnancy, complicated pregnancy, at least one diagnosis of gestational disease, caesarean delivery, induction of labour, resuscitation at birth, admission to neonatal ward and admission to NICU. Increasing gestational age was associated with higher proportions of prematurity of unknown cause or unjustified preterm birth and exclusive BF at discharge. We found no significant differences in social problems and a 5-min Apgar of less than 5 based on gestational age (Table 1).
Analysis of variables by weeks of gestational age at birth.
| Variables | Total | 34 w GA | 35 w GA | 36 w GA | Statistical significance |
|---|---|---|---|---|---|
| Mean birth weight (g) | 2436.96 | 2140.52 | 2376.02 | 2611.46 | p=0.000 |
| Birth weight <10th %ile (%) | 9 | 20.4 | 9.5 | 3.6 | p=0.000 |
| Birth weight >90th %ile (%) | 9.1 | 1.5 | 5.4 | 14.9 | p=0.000 |
| IVF (%) | 13.7 | 17.7 | 13.7 | 12.0 | p=0.000 |
| Multiple pregnancy (%) | 27.7 | 32.3 | 29.2 | 24.5 | p=0.000 |
| Underlying maternal disease (%) | 47.1 | 53.1 | 48.6 | 43.4 | p=0.000 |
| Mean maternal age (years) | 33 | 33 | 33 | 33 | NS |
| Maternal smoking (%) | 9.5 | 12.1 | 8.9 | 8.7 | p=0.000 |
| Social problems (%) | 0.9 | 1.1 | 0.7 | 0.8 | NS |
| Complicated pregnancy (%) | 19 | 28.3 | 21.1 | 13.5 | p=0.000 |
| At least one gestational disease (%) | 41.4 | 55.9 | 43.4 | 33.2 | p=0.000 |
| Caesarean delivery (%) | 47.9 | 56.2 | 46.8 | 44.8 | p=0.000 |
| Planned caesarean (%) | 44.4 | 47.0 | 42.5 | 44.2 | NS |
| Induced labour (%) | 7 | 8.5 | 6.9 | 6.4 | p=0.011 |
| Resuscitation (%) | 10.6 | 17.5 | 11.6 | 6.9 | p=0.000 |
| 5-min Apgar <5 (%) | 0.5 | 0.4 | 0.7 | 0.5 | NS |
| Placental abruption >18h (%) | 18.8 | 27.1 | 18 | 14.9 | p=0.000 |
| Preterm birth of unknown cause (%) | 29 | 21.2 | 29.5 | 33.4 | p=0.000 |
| Preterm birth of unjustified cause (%) | 3.1 | 1.8 | 2.5 | 4.4 | p=0.000 |
| Exclusive breastfeeding (%) | 47 | 41.6 | 44.3 | 51.1 | p=0.000 |
| At least 1 discharge diagnosis (%) | 46.2 | 61.6 | 52.4 | 34.8 | p=0.000 |
| At least 1 procedure (%) | 53.5 | 79.8 | 61.1 | 35.9 | p=0.000 |
| Admission to neonatal ward (%) | 58.6 | 89.5 | 66.8 | 39.2 | p=0.000 |
| Mean stay in neonatal ward (days) | 11 | 14 | 10 | 8 | p=0.000 |
| Intensive care (%) | 15.2 | 24.5 | 17.7 | 9.3 | p=0.000 |
| Mean stay in intensive care (days) | 12 | 13 | 11 | 10 | p=0.001 |
When we compared the variables of sex, maternal age, multiple pregnancy, resuscitation, prenatal exposure to steroids, admission to neonatal ward, mean length of stay and mortality between the LPT infants and FT infants registered in the MPNDS database, we found statistically significant differences in all, with higher values in the LPT group (Table 2).
Analysis of the differences between the sample of late preterm (LPT) infants and the samples of full term (FT) infants obtained from the Minimal Perinatal Dataset (MPNDS) database.
| Variables | LPT | FT (MPNDS) | Statistical significance |
|---|---|---|---|
| Sample size | 9121 | 40,648 | |
| Male sex | 53.6% | 51.7% | p=0.0008 |
| Mean maternal age (years) | 33 (SD, 6) | 32.22 (SD, 5.69) | NS |
| Multiple gestation | 27.7% | 2.3% | p<0.0001 |
| Resuscitation | 10.6% | 0.9% | p<0.0001 |
| Admission to neonatal unit | 58.6% | 11.2% | p<0.0001 |
| Mean stay in neonatal ward (days) | 11 (SD, 11) | 0.61 (SD, 2.71) | p=0.0000 (95% CI, 9.883–1.517) |
| Prenatal steroids | 33.7% | 0.7% | p<0.0001 |
| Death | 2.8‰ | 1‰ | p<0.0001 |
In the period under study, the rate of late preterm birth was 5.9% of live births, making up 70.1% of all preterm births. This means that in Spain alone, during this period, 13,820 LPT deliveries were managed in as few as 35 hospitals. The rates of preterm birth (8.7%) and late preterm birth (5.9%) were consistent with those reported in Europe in recent years, which in turn have been lower than those reported in the United States, which remain at around 8% since year 2000.10,11 Neonatal mortality in PT infants amounts to 2.8‰ of live births, compared to 1‰ in infants born between 37 and 41 weeks’ gestation (based on MPNDS data). In the United States, the mortality of PT infants is high, ranging from 7.4‰ to 9.4‰ of live births, and varies depending on race.11 The impact of PT birth on mortality reaches as far as young adulthood, as the probability of dying between ages 18 and 36 years is much higher in this group compared to individuals born at term.12
The small but significant predominance of male sex in the population under study and in comparison to the MPNDS population is not trivial if we take into account the published evidence on the increased risk of sequelae from longitudinal and neuroimaging studies.13
Our study reflects the factors that have been associated with an increased risk of late preterm birth14: more frequent inductions, the growth in assisted reproductive technologies and the associated increase in multiple pregnancies, maternal pre-existing disease, maternal age and maternal or foetal problems during gestation. The high rate of caesarean delivery (which approaches 50% in Spain, with 18% of caesarean sections performed for an unknown reason, which may include request by the mother) and advanced maternal age (higher maternal age in the LPT births in our study compared to the FT births documented in the MPNDS database) are relevant factors.
When we analysed the reasons for induction before term, we found that maternal or foetal problems accounted for 67.7% of cases. What was the reason in the remaining 33.3%? Was induction justified in all, and were the risks of preterm birth taken into account? In the field devoted to the reason for PT birth, the reason was documented as “unknown” in 29% of LPT cases, which is an acceptable proportion given the usual prevalence of this event. But in an average of 3.1% of LPT deliveries, the reason was clearly identified as “unjustified”, and this proportion increased with increasing gestational age, peaking at 36 weeks. This was the reason documented in 273 of the LPT infants in our sample. The “demand” that the reason for induction or elective caesarean delivery be documented in the health records resulted in a drop in elective deliveries before 39 weeks’ gestation from 26% to 4% within 6 months in a hospital group in the United States.15
Martin et al.16 noted that 30% of LPT deliveries are medically indicated, and wondered whether it would be possible to prevent what has come to be known as “iatrogenic” preterm births. Gyamfi-Bannerman17 highlighted non-evidence based maternal and foetal indications where nonspontaneous early delivery may not be fully justified: mild idiopathic high blood pressure, gestational diabetes, mild cholestasis of pregnancy, oligohydramnios, previous caesarean section and elective caesarean delivery. Thirty-eight percent of pregnant women in our sample had at least one of these conditions. Gyamfi proposed revising the protocols on the obstetric management of premature rupture of membranes, risk of spontaneous preterm delivery and IUGR. New obstetric strategies have proven effective in reducing the rate of elective delivery,18 although it is important to ensure that decreases in the rate of LPT birth are not accompanied by an increase in maternal or foetal mortality.19,20
The incidence of pregnancy in women of advanced age in the general population is 23.66%,21 and in our sample, the mean maternal age at delivery was 33 years (SD, 6 years), with 30.8% of mothers aged more than 35 years. This may have been associated with a higher frequency of IVF (13.4% vs 1–4% in the general population22) and of caesarean delivery. Another salient finding was the high proportion of mothers with pre-existing disease (47.1%) compared to the prevalence reported by one of the participating tertiary care hospitals (29%).23
The pulmonary immaturity of LPT infants compared to FT infants is associated with the need for a greater level of resuscitation and increased frequency of NICU admission. The proportion of admission to neonatal intensive care and the mean length of stay in the NICU are inversely correlated with gestational age, and significantly higher compared to those in FT infants and to published references.24 However, admission protocols for LPT infants vary considerably between hospitals. They range from some that call for routine admission of all LPT infants to others in hospitals with more resources for monitoring and control where a higher proportion of these infants stay with their mothers in the postpartum ward. The need for intensive care is mainly due to respiratory morbidity, which increases with decreasing gestational age. Administration of antenatal steroids in mothers at risk of preterm delivery between 34 and 36 weeks’ gestation has been attempted with promising results in terms of a reduced incidence of respiratory diseases and a decreased need for respiratory support and oxygen, although with an increase in the incidence of hypoglycaemia.25 In the sample under study, 33% of mothers received antenatal steroids.
Hypoglycaemia, found in 30% of LPT infants, and respiratory morbidity, found in 28%, appear to be the factors associated with the unfavourable neurodevelopmental outcomes of LPT infants.26,27 Other conditions that are frequently associated with the need for admission or readmission are hyperbilirubinaemia, IUGR and feeding problems caused by suction difficulties secondary to prematurity, which result in a regrettably low rate of BF in this children (47%) that is inferior to the rate found in FT infants (60–70%).28 We ought to mention the infrequent reference to these problems in our sample (6.4%), when they are surely much more common; it is possible that they are not deemed significant enough to be included in the diagnostic coding.
As expected, the mean length of stay was significantly longer in LPT infants compared to FT infants (11 vs 0.7 days). However, we also need to be concerned about the “healthy” LPT infants, who are discharged early without being considered at risk despite evidence of an increased risk of neurodevelopmental disorders, academic problems and need for intervention in early childhood that may even have an impact on cognition and professional life in adulthood, due to which they should all be properly evaluated.29,30
Rose and Engle31 aptly summarised the key strategies to reduce the rate of late preterm birth: preventing and reducing the number of high-risk pregnancies, avoiding non-medically indicated preterm delivery and considering interventions for the prevention of perinatal morbidity, such as administration of antenatal steroids, transfer of high-risk pregnant women to hospitals with greater care resources, and the implementation of new obstetric protocols for decision-making regarding nonspontaneous preterm delivery. Raju states that while in the past 10 years and after the introduction of the concept of the LPT infant the management of these patients has improved, more needs to be done.32 We need to reinforce the fact that this is a vulnerable population and that we need to continue conducting research with the aim of preventing preterm birth regardless of gestational age.
ConclusionsThe large sample of PT births we analysed allowed us to highlight the increased neonatal morbidity and mortality in this population and their undisputable association with multiple pregnancy, advanced maternal age and the still extended practice of performing labour induction and elective caesarean deliveries without justification.
The results obtained through the SEN34-36/ACUNA database show that we need to continue collecting as much information as possible on late preterm birth in Spain, ensuring the best possible quality of care during the neonatal period, and conveying to health care professionals that since this is a population at risk of experiencing abnormalities in their postnatal development, we must remain alert to identify those cases where it is possible to prevent late preterm birth.
Conflicts of interestThe authors have no conflicts of interest to declare
We thank Luis Miguel Molinero (Alce Ingeniería) and all participating hospitals (ordered by date of inclusion in the database): Hospital (H.) Punta de Europa (Algeciras), H. de Mérida (Badajoz), H. de San Jorge (Huesca), Hospital Universitario (H.U.) Severo Ochoa (Leganés), H. de Zumárraga (Guipúzcoa), H. de Granollers (Barcelona), H. Infanta Margarita (Cabra, Cordoba), H. de Calatayud (Zaragoza), H. Virgen de la Concha (Zamora), H. Infanta Elena (Valdemoro, Madrid), H.U. La Fe (Valencia), H. Santos Reyes (Aranda de Duero, Burgos), H. San Pedro (Logroño), H. Clínic Maternitat (Barcelona), H. de Alcañiz (Teruel), H. Rey Juan Carlos (Móstoles, Madrid), H. de Barbastro (Huesca), H.U. Donostia (Gipuzkoa), SCIAS H. de Barcelona (Barcelona), H. Sant Joan de Déu (Manresa, Barcelona), H. de Igualada (Igualada, Barcelona), H. Clínico U. Lozano Blesa (Zaragoza), H. de la Santa Creu i Sant Pau (Barcelona), H.U. Valme (Seville), H. de la Vega (Murcia), H. del Henares-Coslada (Madrid), USP, Instituto Hispalense de Pediatría. Clínica Sagrado Corazón (Seville), H. Costa de la Luz (Huelva), H. Comarcal de Barbanza (Santiago de Compostela), Clínica Corachán (Barcelona), H.U. Los Arcos del Mar Menor (Murcia), Hospital Central de la Defensa Gómez Ulla (Madrid), H. General de Catalunya (Barcelona), H.U. Reina Sofía (Córdoba), H.U. Virgen de las Nieves (Granada).
Please cite this article as: García-Reymundo M, Demestre X, Calvo MJ, Ginovart G, Jiménez A, Hurtado JA. Prematuro tardío en España: experiencia del Grupo SEN34-36. An Pediatr (Barc). 2018;88:246–252.