Although standard surgical treatment of a testicular tumour is orchiectomy, use can be made of testis-sparing surgery in selected cases, based on tumour markers, tumour size, and histopathological findings. Our objective is to become acquainted with the indications of testis-sparing surgery as a treatment for the incidental finding of a palpable and non-palpable testicular mass.
Material and methodsA retrospective study was conducted on 22 patients younger than 18 years diagnosed with a testicular tumour between 2000 and 2014. An assessment was made of the condition, the history, ultrasound, histopathology, tumour markers (BHCG, AFP), therapeutic approach, and outcome.
ResultsOf the 22 patients (10 prepubertal age) studied, 82% had palpable mass, and 18% were incidental findings. Two had cryptorchidism. The BHCG was increased in 27% and AFP in 45% of cases. There were 18 tumorectomies and 4 orchiectomies performed. The histopathology found 72% germ cell, 14 orchiectomy, and 2 tumorectomies (2 teratomas), with 27% non-germ cell tumours in 4 orchiectomies and 2 tumorectomies (2 cells of Leydig). Six patients received post-surgical chemotherapy (mixed tumours). The median tumour size was 1 (0.4–1.5) cm in tumorectomies, and 2.5 (0.5–14) cm in orchiectomies. The mean follow-up was 5 (1–15) years. One patient died due to metastatic disease. There was no local recurrence in the follow up of the tumorectomies.
ConclusionsA change in the trend of our therapeutic approach is demonstrated. We propose that testis-sparing surgery is indicated in prepubertal patients who meet the benignity criteria of the testicular mass (small size and negative tumour markers).
El tratamiento quirúrgico estándar del tumor testicular es la orquiectomía, sin embargo, se podría recurrir a la cirugía conservadora en casos seleccionados, basándonos en la edad del paciente, marcadores tumorales, tamaño tumoral y hallazgos histopatológicos. Nuestro objetivo es dar a conocer cuáles son las variables que tener en cuenta para indicar una cirugía conservadora como tratamiento de una masa testicular palpable y no palpable encontrada como hallazgo incidental.
Material y métodosEstudio retrospectivo en 22 pacientes menores de 18 años, diagnosticados de tumor testicular entre 2000 y 2014. Revisamos el motivo de consulta, antecedentes, ecografía, estudio histopatológico, marcadores tumorales (BHCG, AFP), actitud terapéutica y evolución.
ResultadosDe los 22 pacientes (10 prepuberales), el 82% presentaron masa palpable y el 18% fueron hallazgos incidentales. Dos presentaban criptorquidia. La BHCG estaba aumentada en el 27% y la AFP en el 45%. Se realizaron 18 orquiectomías y 4 tumorectomías. La histología fue en un 72% de células germinales, 14 orquiectomías y 2 tumorectomías (2 teratomas); y en un 27% de tumores de células no germinales, en 4 orquiectomías y 2 tumorectomías (2 tumores de células de Leyding). Seis pacientes recibieron quimioterapia postoperatoria (tumores mixtos). La mediana del tamaño de la tumoración fue de un cm (0,4-1,5) en las tumorectomías y de 2,5cm (0,5-14) en las orquiectomías. El seguimiento fue de 5 años (1-15). Un paciente falleció por enfermedad metastásica. No hubo recidiva local en la evolución de las tumorectomías.
ConclusionesPonemos de manifiesto una tendencia al cambio en nuestra actitud terapéutica. Planteamos una cirugía conservadora mediante tumorectomía en los pacientes que cumplan con los criterios de benignidad de la masa testicular (pequeño tamaño y marcadores tumorales negativos).
Testicular tumours account for 1–2% of all paediatric solid tumours, with 2 peaks in age of onset: the first one at ages 2–4 years, and the second at around age 15 years.1 Its incidence in the general population is of 0.5–2 cases per 100,000 inhabitants, with an increase in the adult population in recent years.2,3
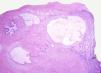
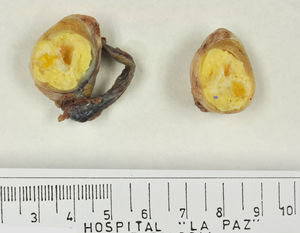
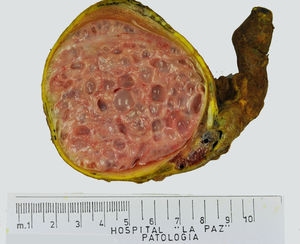
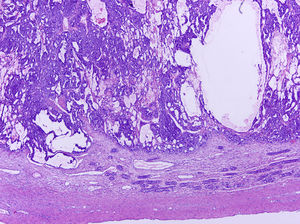
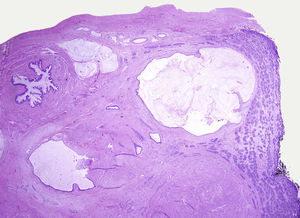
Testicular tumours are divided into 6 groups (World Health Organization histological classification of tumours, 2016)4: (1) germ-cell tumours derived from germ cell neoplasia in situ (GCNIS) including seminoma, embryonal carcinoma, postpubertal-type yolk sac tumour, trophoblastic tumour and germ cell tumours of unknown type (‘burnt-out’ tumours); (2) germ cell tumours unrelated to GCNIS, including spermatocytic tumour, prepubertal-type teratoma, prepubertal-type yolk sac tumour and prepubertal-type mixed teratoma and yolk sac tumour; (3) sex-cord-stromal tumours (Leydig cell, Sertoli cell or granulosa cell tumours); (4) tumours containing both germ cell and sex cord-stromal elements; (5) miscellaneous tumours of the testis and (6) haematolymphoid tumours. This new classification differentiates between 2 types of teratomas (Figs. 1 and 2) and yolk sack tumours: prepubertal type (unrelated to GCNIS) and postpubertal type (derived from GCNIS), with the prognosis being significantly better in the former. There is a clear predominance (90–95%) of germ cell tumours, and prepubertal-type teratomas and yolk sac tumours are the most frequent testicular tumours in children5–8 (Fig. 3). Prepubertal-type teratomas are usually cystic and have an organoid arrangement (Fig. 4). This type includes epidermoid cysts, dermoid cysts and well-differentiated neuroendocrine tumours.
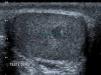
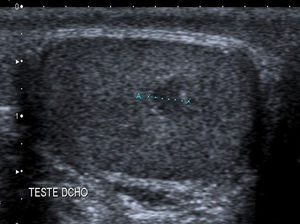
Due to the routine use of ultrasound examination, there has been an increase in the detection of cases of nonpalpable testicular masses9,10 (Fig. 5).
Radical orchiectomy has been traditionally considered the gold standard of treatment for testicular masses at any age. However, given that these masses are frequently benign in children,1,10,11 the medical and surgical approach to their management has been re-evaluated in recent years. This involves a change in the initial treatment approach, with consideration of conservative surgery (tumourectomy)11–13 based on tumour marker values (beta-hCG, AFP), tumour size and histological findings.14–19
The decision of performing a conservative tumourectomy must be made jointly by the physicians in charge of the patient (Table 1), and the intervention must be performed in a centre with experience.2
Summary of clinical cases.
| Case | Age (years) | Risk factors | Presentation | Tumour markers (beta-hCG/AFP) | Surgical approach | Tumour size (cm) | Histology |
|---|---|---|---|---|---|---|---|
| 1 | 18 | Enlargement | N/P | Orchiectomy | 2.7 | Mixed germ cell | |
| 2 | 15 | Enlargement | P/P | Orchiectomy | 14 | Mixed germ cell | |
| 3 | 16 | Enlargement | P/N | Orchiectomy | 2.5 | Mixed germ cell | |
| 4 | 0.16 | Enlargement | X/P | Orchiectomy | 1 | Granulosa cell | |
| 5 | 2 | Enlargement | N/P | Orchiectomy | 2.5 | Yolk sac | |
| 6 | 16 | Enlargement | P/P | Orchiectomy | 3.5 | Mixed germ cell | |
| 7 | 12 | Ultrasound finding | N/N | Tumourectomy | 1 | Leydig cell | |
| 8 | 0.83 | Enlargement | N/N | Tumourectomy | 1.5 | Prepubertal-type teratoma | |
| 9 | 6 | Enlargement | P/P | Orchiectomy | 4.5 | Mixed germ cell | |
| 10 | 14 | Enlargement | X/N | Orchiectomy | 1.5 | Prepubertal-type teratoma | |
| 11 | 16 | Enlargement | N/N | Orchiectomy | 6.5 | Prepubertal-type teratoma | |
| 12 | 6 | Ultrasound finding | N/N | Orchiectomy | 1.5 | Prepubertal-type teratoma | |
| 13 | 11 | Cryptorchidism | Ultrasound finding | N/N | Tumourectomy | 0.4 | Leydig cell |
| 14 | 12 | Enlargement | N/N | Orchiectomy | 0.5 | Non-Hodgkin lymphoma | |
| 15 | 0.91 | Enlargement | N/P | Orchiectomy | 1.5 | Yolk sac | |
| 16 | 18 | Enlargement | P/N | Orchiectomy | 4 | Mixed germ cell | |
| 17 | 16 | Enlargement | N/P | Orchiectomy | 2.4 | Postpubertal-type teratoma+GCNIS | |
| 18 | 2 | Enlargement | N/N | Orchiectomy | 1.5 | Prepubertal-type teratoma | |
| 19 | 13 | Enlargement | N/N | Tumourectomy | 1.5 | Teratoma | |
| 20 | 10 | Enlargement | P/P | Orchiectomy | 9 | Mixed germ cell | |
| 21 | 12 | Cryptorchidism | Surgical pathology | X/X | Orchiectomy | 5 | Sertoli |
| 22 | 0.25 | Enlargement | X/P | Orchiectomy | 3 | Granulosa cell |
GCNIS, germ cell neoplasia in situ; N, negative; P, positive; X, not measured.
Our objective was to determine the variables that need to be taken into account in the indication of conservative surgery for treatment of palpable and nonpalpable testicular masses found incidentally.
Materials and methodsWe conducted a retrospective study of cases of testicular tumours in 22 patients aged less than 18 years diagnosed and managed in our hospital between 2000 and 2014. We excluded cases in patients with gonadal dysgenesis, as they require a different treatment and followup. We analysed the age at diagnosis, clinical presentation, ultrasound findings, histopathological findings, tumour marker values (beta-hCG, AFP), therapeutic approach (orchiectomy vs tumourectomy) and followup, which was performed as needed in the urology and oncology clinics.
ResultsThe mean age of the 22 patients was 9.6 years (range, 2 months-18 years), 10 cases were diagnosed prepubertally. As for the clinical presentation, a palpable mass was the most frequent finding, in 18 (82%) of cases, 2 of which were diagnosed in the context of a routine paediatric checkup. In 4 cases (18%), the diagnosis resulted from an incidental finding, 3 of ultrasound examinations ordered for evaluation of short stature, orchiodynia and contralateral cryptorchidism, and 1 in the examination of a surgical specimen from a patient that had undergone surgery for cryptorchidism with removal of an atrophied testis.
Two patients had a history of cryptorchidism, bilateral in 1, and contralateral to the tumour in the other.
During the diagnostic evaluation, the level of beta-hCG was measured in 18 patients (82%), and the level of AFP in 21 (95%), and they were found to be elevated in 27% and 45% of tested patients, respectively.
An ultrasound examination was performed in 90% of patients. The median size of the palpable masses was 3.5cm (1.2–7) and the median size of nonpalpable masses was 1cm (0.4–1.5).
A total of 18 orchiectomies and 4 tumourectomies were performed in our patients. The approach was through an inguinal incision in all. The indication for tumourectomy was based on tumour size (<2cm) and negative results of tests for tumour markers.
The distribution by tumour histology was: 72% of germ cell tumours, treated with 14 orchiectomies (7 mixed germ cell tumours [6 with a choriocarcinoma or embryonal carcinoma component], 5 teratomas, 2 yolk sac tumours) and 2 tumourectomies (2 teratomas); and 27% of non-germ cell tumours, treated with 4 orchiectomies (2 granulosa cell tumours, 1 Sertoli cell tumour, 1 non-Hodgkin lymphoma) and 2 tumourectomies (2 Leydig cell tumours). The median tumour size was 1cm (0.4–1.5) in the cases treated with tumourectomy and 2.5cm (0.5–14) in the cases treated with orchiectomy.
Elevation of beta-hCG at diagnosis corresponded to choriocarcinomatous tumours, and elevation of AFP to tumours with a yolk sac component.
The followup of these patients consisted of visits in our clinic at 1 week, 1 month and 6 months post surgery, and yearly thereon. In every visit, patients underwent a testicular ultrasound examination to assess parenchymal morphology and a blood test with measurement of tumour markers to ascertain their decline. All patients were referred to the Department of Haematology and Oncology, whose staff determined how patients should be managed and the need for followup in the department's clinics based on the histopathological findings.
Six patients, all with mixed germ cell tumour histology, received postoperative chemotherapy consisting of 2 cycles of bleomycin/etoposide/cisplatin. During the followup, tumour marker levels because negative in all patients except one that died of metastatic disease. The duration of followup was 5 years (1–15); 4 patients remain in followup per protocol in the hospital that was the source of referral.
One patient died; he had a mixed germ cell tumour (with a choriocarcinoma component comprising 50%) and developed metastases in the lung and liver, bone marrow failure secondary to chemotherapy and septic shock. None of the other patients experienced local recurrence or metastasis during the followup. Only 1 patient underwent surgery for insertion of a testicular prosthesis.
DiscussionTesticular tumours are infrequent in the paediatric age group. The epidemiologic risk factors for the development of testicular tumours at any age describe in the literature are: history of cryptorchidism, Klinefelter syndrome, history of testicular cancer in first-degree relative, presence of contralateral tumour, and infertility.2 In our sample, out of all these risk factors, we only found cryptorchidism in 2 patients.
In our study, the most frequent presentation was palpable mass (82%), which was consistent with the previous literature.5,10 The tumour was found by ultrasound examination in 13% of cases. The widespread use of sonography has led to an increase in the incidental detection of small testicular masses of less than 2cm in diameter, defined as nonpalpable scrotal masses, and considered benign in 80% of cases,12 which can consequently be treated with conservative surgery. In our sample, the postoperative histopathological examination confirmed the benign nature of these nonpalpable masses.
As for tumour markers, we found evidence that AFP is elevated (> 10ng/mL) in 92–100% of patients with yolk sac tumours and that beta-hCG may be elevated in germ cell tumours (with mild elevation in embryonal carcinomas or seminomas containing syncytiotrophoblastic giant cells, and marked elevation in cases with a choriocarcinoma component).3 In our study, at the time of diagnosis, AFP was elevated in 45% of cases (every yolk sac tumour and tumours with a teratoma component) and beta-hCG in 27% (every tumour with a choriocarcinoma component or with syncytiotrophoblastic giant cells). The levels of tumour markers normalised during the followup except in 1 patient that died of advanced metastatic disease. For the purposes of diagnosis and followup, it is important to consider that patients aged less than 12 months may exhibit a physiological elevation of AFP, with levels of up to 100ng/mL.8
The histology of testicular tumours varies depending on the cell line from which they originate. Germ cell tumours are the most frequent type, amounting to 95% of total cases in adults and 65% of cases in the paediatric age group.5 In our series, germ cell tumours constituted 75% of the sample.
Teratomas are usually benign in prepubertal patients, so they may be treated with tumourectomy in cases where ultrasound examination reveals viable normal testicular tissue and with normal levels of AFP, or, in infants aged less than 12 months, elevated AFP levels that do not exceed 100ng/mL.8 Yolk sac tumours are the most frequent type of malignant tumour in children, and, like embryonal carcinomas, usually develop before age 2 years.5–8 They are treated with radical orchiectomy. Radical retroperitoneal lymph node dissection is performed routinely in adult patients, but in children this depends on staging, as most cases in prepubertal patients (85%) are stage I. At present, the lymph nodes are not routinely removed, except in patients with more advanced disease. Stromal tumours are rare in the prepubertal age group and may be associated with precocious puberty. The tumours that recur most frequently are Sertoli cell tumours, whose incidence peaks at age 6 months. Thirty percent of Sertoli cell tumours are associated to endocrine syndromes or abnormalities (Peutz-Jeghers syndrome, Carney complex, Cushing disease, pituitary adenomas). Tumour resection is usually curative in children.2 Malignant cases are rare and usually occur in older children, and therefore performance of radical orchiectomy with postoperative followup is recommended in patients aged 5 years and older.10 Leydig cell tumours develop between ages 5 and 10 years, are generally benign and tumour resection is usually curative. Juvenile granulosa cell tumours usually appear in the first year of life, most commonly in the first 6 months; it is the most frequent testicular tumour in newborns and has a good prognosis in this age group, unlike granulosa cell tumours that develop in postpubertal patients.10
Orchiectomy was the surgical approach used in 18 patients (82% of our sample). Six of them (5 of postpubertal age), all of who had mixed tumours with a choriocarcinoma or embryonal carcinoma component, received postoperative chemotherapy consisting of 2 cycles of bleomycin/etoposide/cisplatin.
A conservative surgical approach with tumourectomy has been performed in our hospital since 2009 and was the approach used in 18% of our sample. The decision to change the approach to the management of testicular masses was motivated by the possibility of sparing a large amount of testicular tissue thanks to the benign course of the disease in the prepubertal age group, the higher prevalence of favourable histology, and the low probability of recurrence.1,3,7–11
We conducted a retrospective analysis of cases in a sample of our patients and found that 3 patients could have been treated with tumourectomy instead, as they met the criteria for management with this procedure (prepubertal, negative tumour markers, tumour size<2cm).
One of the patients died during the followup of advanced disease with distant metastasis. We found no other cases of recurrence in either the orchiectomy or the tumourectomy, which demonstrates the high cure rate in both patients with favourable histology and patients with unfavourable histology.2,12,14–19
In conclusion, testicular masses in prepubertal patients are different from those in postpubertal patients, as they have a different epidemiology, histology and prognosis. Therefore, the approach to their management should not be the same: we can consider a conservative surgical approach with tumourectomy in patients that meet the benign testicular tumour criteria (small size and negative tumour markers).
Conflicts of interestThe authors have no conflicts of interest to declare
Please cite this article as: Romo Muñoz MI, Núñez Cerezo V, Dore Reyes M, Vilanova Sánchez A, González-Peramato P, López Pereira P, et al. Tumores testiculares en la edad pediátrica: indicaciones de la cirugía conservadora. An Pediatr (Barc). 2018;88:253–258.