Early-onset neonatal sepsis refers to an infection which starts during the first 72h of birth, and can lead to significant morbidity and mortality. Scientific evidence shows that infected infants present with symptoms during the first hours after delivery. There has been a significant decrease in this condition with the implementation of guidelines for its prevention. However, international guidelines still recommend the evaluation of these infants using painful tests.
Material and methodsA prospective cohort study was conducted on all asymptomatic infants born at >35 weeks gestation with one or more risk factors in a single tertiary care centre from 2011 to 2015. They were periodically observed in newborn nursery from admission until discharge looking for signs of infection.
ResultsOut of the 9,424 babies born during this period, 1,425 were included in the study. A total of 53 infants were admitted to the neonatal unit, half of them because of sepsis suspicion. Finally, just 7 were discharged with the diagnosis of sepsis. All these 7 presented with symptoms during their first 72h of life. No sepsis was reported in asymptomatic infants.
ConclusionsTruly infected infants present with symptoms during their first hours of life. This study supports the observation of infants at risk as a safe practice to detect early-onset sepsis.
La sepsis vertical precoz es una causa importante de morbimortalidad neonatal. La evidencia científica apunta a que la mayoría de los recién nacidos infectados presentan síntomas en las primeras horas de vida. Tras la aplicación de las medidas para la prevención de sepsis vertical y el descenso en su incidencia, se han propuesto cambios en el manejo de estos niños. No obstante, la realización de exploraciones complementarias dolorosas aún sigue siendo una práctica muy extendida.
Material y métodosEstudio prospectivo realizado entre 2011 y 2015. Se incluyó a todos los recién nacidos con edad gestacional ≥ 35 semanas, asintomáticos al nacimiento que presentaban uno o más factores de riesgo infeccioso. Durante su estancia en maternidad se realiza observación clínica periódica para la detección de síntomas compatibles con infección.
ResultadosDe los 9.424 recién nacidos en este periodo, 1.425 cumplían los criterios de inclusión del estudio; 53 pacientes precisaron ingreso, la mitad de ellos por sospecha de infección, confirmándose finalmente solo en 7 este diagnóstico. Todos los pacientes presentaron clínica en las primeras 72 h de vida.
ConclusionesLos niños con factores de riesgo infeccioso que desarrollan una infección presentan clínica de forma precoz en las primeras horas tras el nacimiento. Este trabajo apoya la observación clínica estrecha como medida suficiente y segura para la detección de la sepsis neonatal precoz.
Based on the criteria adopted by the Grupo de Hospitales Castrillo (Castrillo Hospital Group [GHC]), systemic inflammatory response syndrome (SIRS) is defined as the presence of clinical manifestations (toxic appearance and/or 2 or more symptoms or signs of infection) and positive laboratory tests, and neonatal sepsis as presence of SIRS with evidence of infection, that is, isolation of a pathogen from blood culture. Similarly, based on the criteria of this working group, we define vertically transmitted neonatal sepsis as infection within 72h of life where nosocomial infection has been ruled out.1,2 This is a significant cause of neonatal morbidity and mortality.3–5 Historically, the most frequent aetiological agent has been group B streptococcus (GBS) or Streptococcus agalactiae (30–50%), followed by Escherichia coli (E. coli) (26%).6,7 However, based on the 2015 data presented at the meeting of the GHC in 2016 whose publication is still pending, E. coli (35.7%) has overtaken GBS (31.4%) as the main causative agent of vertically transmitted early-onset sepsis. Other pathogens, such as Staphylococcus aureus, Enterococcus faecalis and Listeria monocytogenes, are involved less frequently.2,5
In the 1970s, the incidence of early-onset neonatal sepsis associated with GBS in the absence of preventive interventions was as high as 3 cases per 1,000 births. Following the publication of the recommendations for the prevention of perinatal group B streptococcal disease by the Centers of Disease Control and Prevention (CDC) in 1996 and their 2002 and 2010 updates and the introduction of intrapartum antibiotic prophylaxis in cases with risk factors, we have witnessed a decrease in the incidence of sepsis of any aetiology, and especially of sepsis caused by GBS.1,8 However, GBS is still a frequent cause of sepsis in term and late preterm newborns.
At present, the incidence of vertically transmitted early-onset sepsis in countries that implement preventive measures is of around 0.9–1 per 1,000 live births, with an incidence of confirmed vertically-transmitted sepsis (with positive cultures) of 0.76–0.9 per 1000 live births.9,10 In Spain, based on data of the GHC, there has been a marked decrease from 1.25 per 1,000 live births in 1996 to approximately 0.7 per 1,000 live births in 2005, with a spike of 0.96 per 1,000 births in 2015 (unpublished data).11
Thanks to the introduction of preventive measures, mortality has dropped from 50% in the 1970s to 4–5% of affected full-term newborns at present.12 This illness is associated with significant morbidity, and patients may develop neurologic sequelae or sensory impairment.13,14
The causative microorganisms are commensal bacteria of the gastrointestinal tract, which is the main reservoir and from which bacteria can spread to the vaginal cavity. Maternal colonisation is the main risk factor for the development of vertically transmitted sepsis. The presence of bacteria in the urine at any point during gestation is associated with an increased risk of neonatal sepsis, as it is indicative of significant maternal colonisation. It is estimated that overall, between 10% and 30% of women carry GBS during pregnancy; the prevalence of colonisation by other bacteria is unknown since screening for other pathogens is not performed routinely.7 In Spain, the documented rate of colonisation ranges between 10% and 18%.6 The mechanism of vertical transmission involves infection of the newborn by pathogens from the genitourinary tract of the colonised mother. It may occur through spread of the pathogen to and through the ruptured membranes during labour, invasion of the intact membranes, or exposure during the passage of the child through the birth canal.15
There are other risk factors besides maternal colonisation, such as preterm birth (gestational age [GA] <37 weeks), prolonged rupture of membranes (≤18h), and maternal fever (temperature >38°C), possibly secondary to chorioamnionitis.16
Although they may be subtle and nonspecific, the clinical signs of infection appear in the first 24h of life in 90% of affected newborns, so close observation during this period is of the essence.9
Due to the associated risks, numerous committees and renowned institutions have traditionally recommended performance of painful diagnostic procedures (collection of blood sample for white blood cell count with differential and measurement of acute phase reactants) in newborns with risk factors for infection.17 The laboratory tests performed in at-risk patients have a low sensitivity and specificity, and attempts to establish clinical prediction rules for the early identification of newborns at high risk of sepsis have been unsuccessful, circumstances that may lead to unnecessary hospital admission for initiation of antibiotherapy. This requires the separation of the mother-child dyad, hindering the establishment of breastfeeding, and involves performance of painful diagnostic tests and initiation of treatments that may cause adverse events. Performance of isolated diagnostic tests is not recommended, since, for instance, the positive predictive value of the white blood cell count is as low as 37%, and the sensitivity of the immature to mature neutrophil ratio is 46%. The positive predictive value of sepsis screening panels in neonates is of less than 30%; however, the negative predictive accuracy in the first 12h of birth has been high (>99%) in small clinical studies.17,18
After the implementation of preventive measures against vertically transmission sepsis and the subsequent decrease in its incidence, changes to its management have been proposed. Notwithstanding the recommendations of some scientific societies, the evidence shows that most newborns that develop sepsis do so within 48h of birth. The observation approach is not associated with increases in the delay of treatment, morbidity or mortality compared to performance of diagnostic tests. Thus, close observation may suffice to identify the newborns that develop early-onset sepsis. This approach would also prevent the separation of mother and child, changes to the intestinal microbiota and the activation of the immune system associated with antibiotic treatment, should the latter not be necessary.
The measures used to prevent early-onset sepsis have not been effective in preventing late-onset neonatal sepsis, whose incidence remains stable at around 0.3–0.4 per 1000 live births. The underlying mechanism of infection in these cases remains unclear.19,20
In light of all of the above, we decided to implement a clinical observation protocol in newborns at risk of infection to avoid separating mother and child and reduce performance of painful procedures and unnecessary antibiotic treatment.
Patients and methodsWe conducted a prospective study between March 2011 and May 2015 in the maternity ward of a tertiary level university hospital that manages an average of 2,200 deliveries a year.
The maternity ward has 25 single and 3 double patient rooms.
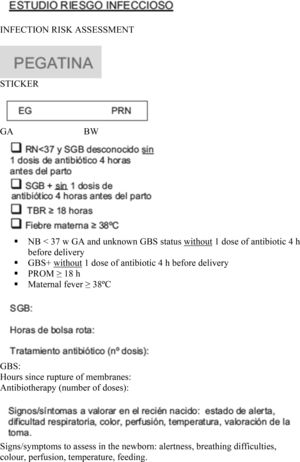
We included all newborns delivered at or after 35 weeks’ gestation at risk of infection who were asymptomatic at birth. During the stay in the maternity ward, a standardised form was used to collect data on the risk factors for infection and the demographic characteristics of the newborns, the clinical assessment of the paediatrician (at 2h, at 6–18h and thereon daily until discharge), need for admission, performance of diagnostic tests, and age at discharge (in hours). We considered the following risk factors for infection: birth before 37 weeks’ gestation, unknown maternal GBS status, GBS+ mother and no antibiotic prophylaxis or incomplete prophylaxis (defined as 1 dose of antibiotic less than 4h before birth), rupture of membranes lasting 18h or longer, and maternal fever of more than 38°C (Appendix A).
We used the following definitions:
- –
Confirmed or highly probable vertically transmitted sepsis: case with compatible symptoms and laboratory findings with onset within 72h from birth where nosocomial infection has been ruled out and with positive blood or neonatal surface culture.
- –
Clinical vertically transmitted sepsis: sepsis with onset within 3 days from birth and with presence of SIRS in the newborn, with negative blood culture results but other data suggestive of an infectious aetiology (risk factors for vertical transmission and/or isolation of pathogenic microbes from the maternal birth canal or neonatal surface exudates in the early hours of life).1
We also reviewed the data for newborns delivered at or after 35 weeks’ gestation admitted to the neonatal unit during the study period with a discharge diagnosis of confirmed vertically transmitted early-onset neonatal sepsis, assessing the presence of risk factors for infection.
The data was analysed with the software SPSS version 22.0 for Mac. We verified the normal distribution of quantitative variables by means of the Kolmogorov–Smirnov test. We have expressed normally distributed variables as mean±standard deviation (SD) and qualitative variables as the absolute frequencies and percentages for each category. We analysed the association between qualitative variables by means of the chi square test.
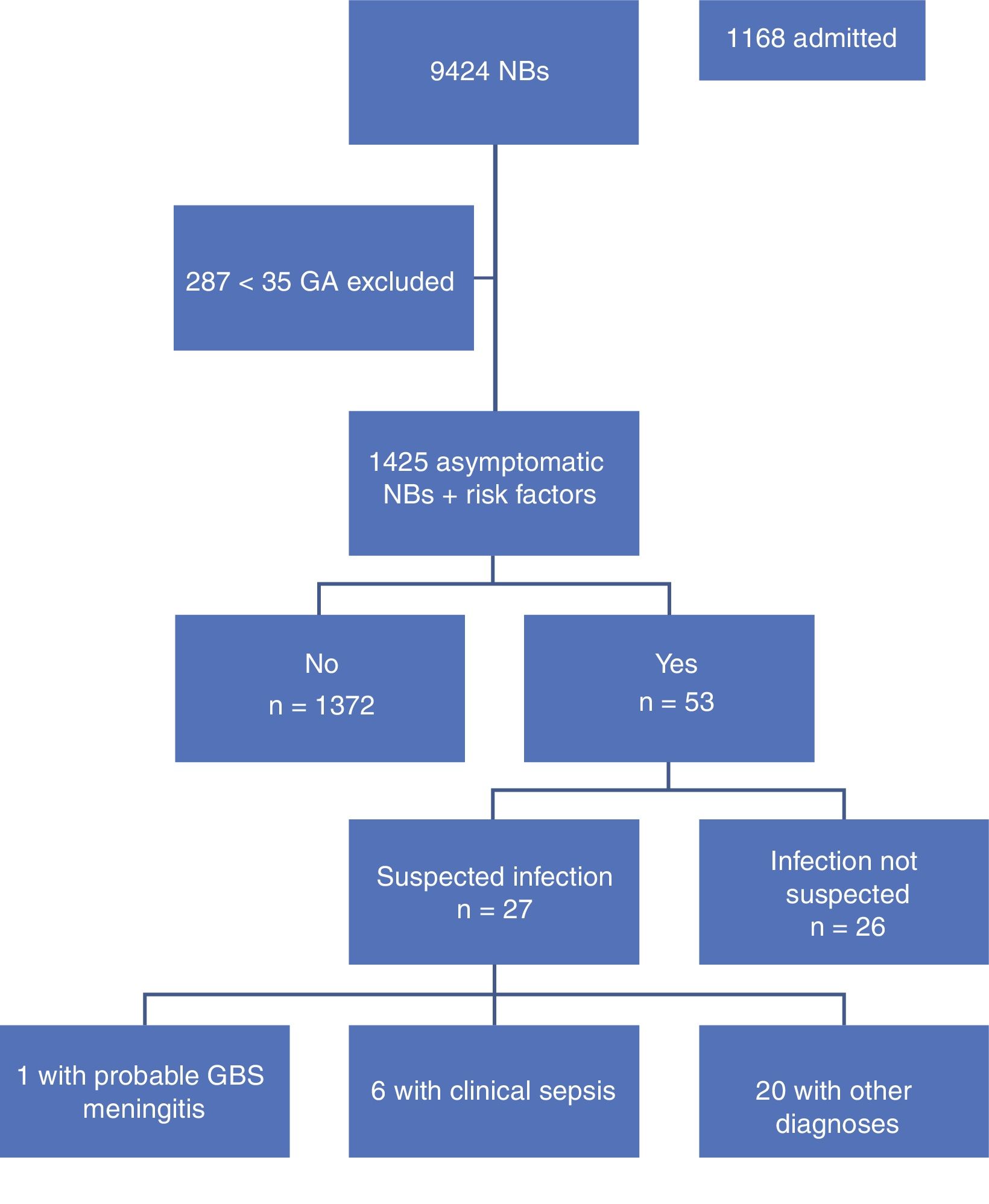
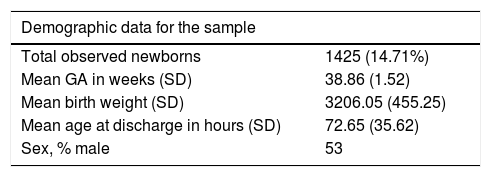
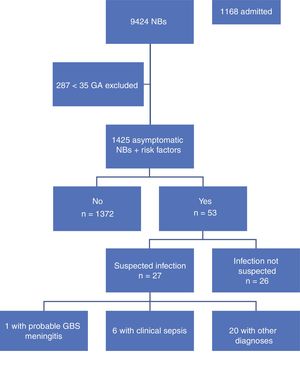
ResultsA total of 9,424 children were born in the period under study, of who 9,137 (96%) were 35 weeks or more of GA. Our study included 1425 newborns (15.6%) that were asymptomatic at birth and met the inclusion criterion of having one or more risk factors. Table 1 summarises the demographic characteristics of the sample. Fig. 1 presents a flowchart of the distribution of the children born in the period under study.
The most frequent risk factor that led to inclusion in the study was prolonged rupture of membranes lasting 18 or more hours (63%, n=899), followed by positive GBS test in the mother without intrapartum antibiotic prophylaxis or with incomplete prophylaxis (22.9%, n=327), maternal fever of 38°C or higher during delivery (11.3%, n=161), and lastly preterm birth with mother of unknown GBS status (7%, n=100). Of all the newborns included in the study, 61 (4.3%) had 2 or more risk factors.
A total of 958 mothers (67.2%) received intrapartum antibiotic prophylaxis. Different antibiotic regimens were used, the most frequent being intravenous penicillin or ampicillin (88.83%, n=851).
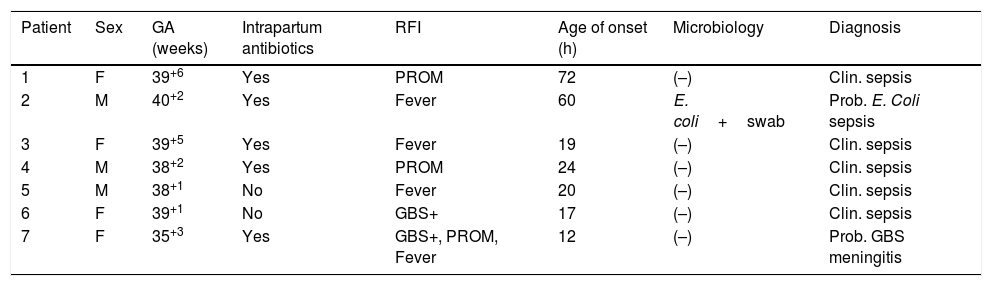
During the period under study, 1372 of the included newborns (96.2%) were discharged home from the maternity ward after a mean stay of 72.65h. Four were discharged early, less than 48h after birth, and were evaluated at the hospital within 24h from discharge, at which time they all remained asymptomatic. The other 53 newborns (3.8%) developed symptoms during the observation period, so they were admitted to hospital with performance of diagnostic tests. In 26 (1.82%) of admitted newborns, the reason for admission was not suspected infection but a different problem, such as jaundice or hypoglycaemia. The remaining 27 (1.89%) were admitted with symptoms suggestive of infection; after performance of the appropriate diagnostic tests, infection was ruled out in 20 (1.4%) and sepsis diagnosed in only 7 (0.49%), corresponding to 6 cases of clinical sepsis without culture confirmation and 1 case of meningitis due to GBS (Table 2).
Characteristics of the patients admitted to hospital that had a discharge diagnosis of sepsis.
| Patient | Sex | GA (weeks) | Intrapartum antibiotics | RFI | Age of onset (h) | Microbiology | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | F | 39+6 | Yes | PROM | 72 | (–) | Clin. sepsis |
| 2 | M | 40+2 | Yes | Fever | 60 | E. coli+swab | Prob. E. Coli sepsis |
| 3 | F | 39+5 | Yes | Fever | 19 | (–) | Clin. sepsis |
| 4 | M | 38+2 | Yes | PROM | 24 | (–) | Clin. sepsis |
| 5 | M | 38+1 | No | Fever | 20 | (–) | Clin. sepsis |
| 6 | F | 39+1 | No | GBS+ | 17 | (–) | Clin. sepsis |
| 7 | F | 35+3 | Yes | GBS+, PROM, Fever | 12 | (–) | Prob. GBS meningitis |
F, female; GA, gestational age; GBS, group B streptococcus; M, male; PROM, prolonged rupture of membranes; RFI, risk factors for infection.
Out of the 61 newborns with 2 or more risk factors for infection, 10 (9.8%) were admitted to hospital, 6 of them (0.42%) for suspected infection. Thus, 13.39% of newborns with 2 or more risk factors were admitted compared to 3.15% of newborns with only 1 risk factor, a difference that was statistically significant (P<.05).
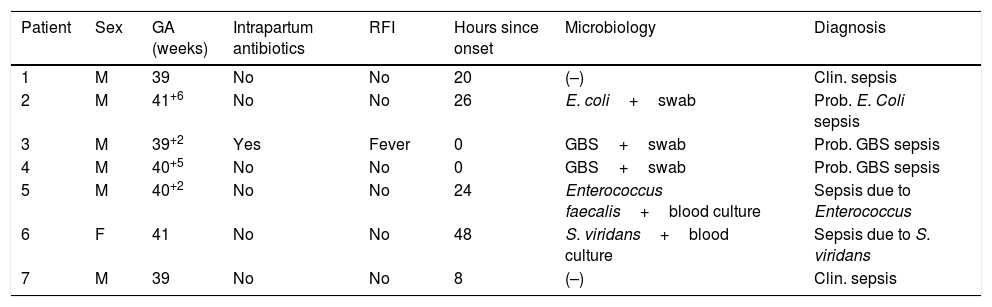
During the study period, 1168 newborns were admitted to the neonatal unit, of who 7 (0.59%) received a diagnosis of confirmed early-onset sepsis, corresponding to an incidence of proven sepsis of 0.7 per 1000 live births. Within this subgroup, the isolated pathogen was GBS in 3 cases (0.25%), corresponding to an incidence of 0.32 per 1000 live births. Of all admitted patients with confirmed sepsis, 6 had no risk factors for infection. Table 3 shows the characteristics of the patients admitted for suspected infection that received a diagnosis of clinical or confirmed sepsis.
Characteristics of patients with a sepsis diagnosis that did not meet the inclusion criteria.
| Patient | Sex | GA (weeks) | Intrapartum antibiotics | RFI | Hours since onset | Microbiology | Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | M | 39 | No | No | 20 | (–) | Clin. sepsis |
| 2 | M | 41+6 | No | No | 26 | E. coli+swab | Prob. E. Coli sepsis |
| 3 | M | 39+2 | Yes | Fever | 0 | GBS+swab | Prob. GBS sepsis |
| 4 | M | 40+5 | No | No | 0 | GBS+swab | Prob. GBS sepsis |
| 5 | M | 40+2 | No | No | 24 | Enterococcus faecalis+blood culture | Sepsis due to Enterococcus |
| 6 | F | 41 | No | No | 48 | S. viridans+blood culture | Sepsis due to S. viridans |
| 7 | M | 39 | No | No | 8 | (–) | Clin. sepsis |
F, female; GA, gestational age; GBS, group B streptococcus; M, male; RFI, risk factors for infection.
Universal antenatal GBS screening in pregnant women and intrapartum antibiotic prophylaxis in deliveries with an identified risk of vertical transmission of infection to the newborn have led to a drastic reduction in the incidence of neonatal sepsis in Spain. Thus, it is reasonable to consider changing the approach to the management of at-risk newborns.21 According to the management guidelines published by the CDC and the American Academy of Paediatrics in 2011, most newborns, although not all, could be managed with clinical observation alone.8,17
Several studies have demonstrated the ineffectiveness of diagnostic tests in comparison to the very strong negative predictive value of remaining asymptomatic despite the presence of risk factors for infection.22 We also ought to highlight that while most newborns that develop early-onset sepsis have risk factors for infection, up to one fourth of them do not have any.7 In our study, we considered the risk factors for infection already published in the guidelines of the CDC and other internationally renowned institutions, such as NICE, which we already specified in previous sections of this article.
We only recruited patients born at a GA of 35 or more weeks, as has been done in previous studies, since these children are at lower risk of developing severe sepsis. We identified risk of sepsis in 15.6% of the included newborns due to the presence of one or more risk factors, a percentage that was very similar to those reported by other authors.21 As has been noted previously in the published literature, the presence of 2 or more risk factors for infection is associated with an increased risk of developing sepsis, which was confirmed in our study.9
However, of all the newborns admitted to hospital with a diagnosis of sepsis, 42% had no risk factor for infection, exceeding the proportion of 25% reported in the literature.7
We found an incidence of confirmed vertically transmitted sepsis of 0.7 per 1,000 live births, which was consistent with the figures reported for Spain overall.9,21 Similarly, the incidence of sepsis due to GBS of 0.32 per 1000 births that we found in our study was consistent with the data published in the CDC guidelines (0.34–0.37‰) and previous data of the CHG (0.31‰).6,12
Of the 14 newborns admitted for suspected sepsis, 12 (85.7%) developed symptoms in the first 48h of life, and the other 2 (14.28%) before 72h from birth, which is consistent with the percentages published in previous works.9
Given all that has been discussed this far, it stands to reason that we need to find a balance weighing the risk of failing to diagnose a potentially severe disease as is early-onset sepsis against the performance of diagnostic tests, hospital admission and administration of intravenous antibiotherapy to healthy newborns that just happen to have risk factors and that remain asymptomatic at all times.21 The presence of risk factors is simply that, an increased risk, which does not necessarily mean sepsis will develop. No risk factor has proven reliable enough for distinguishing those newborns that go on to develop sepsis.10
A study that collected data on newborns delivered at or after 35 weeks’ gestation found that performance of diagnostic tests in asymptomatic newborns provided information of little use in their management, and the authors remarked that better methods to detect newborns actually at risk of infection need to be developed.21
There is also evidence of long-term side effects of the use of antibiotics. A study conducted in Sweden showed that antibiotic exposure in the first week of life is associated with an increased risk of recurrent wheezing in the past 12 months through age 4.5 years. Exposure to antibiotics also alters the intestinal microbiota.23–25
The literature shows that the positive and negative predictive values of isolated laboratory tests for the detection of vertically transmitted sepsis are low. Furthermore, clinical manifestations have a high sensitivity but a poor positive predictive value. Nevertheless, newborns with sepsis usually develop symptoms within 48h of birth, albeit nonspecific ones, even in cases where intrapartum antibiotic prophylaxis has been performed.9
There is unanimous agreement that newborns presenting with symptoms compatible with sepsis should receive empirical antibiotherapy regardless of the presence or absence of risk factors. What remains to be clarified is the approach that should be taken in asymptomatic newborns with risk factors for infection. Recommendations for the management of newborns at risk of infection were published in Switzerland in 2013, in line with the guidelines of the American Academy of Pediatrics, calling for close observation of at-risk newborns during the first 48h life based on evidence that 90% of newborns with early-onset sepsis develop symptoms within this window.
Based on the results of our study and the existing literature, clinical observation seems to be a sufficient and safe method for the detection of early-onset neonatal sepsis, avoiding the separation of mother and child and the performance of diagnostic tests and reducing antibiotic use. Performance of further research would be desirable to allow working groups of scientific societies to review current protocols in order to validate and routinely implement these practices.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Escribano García C, del Mar Montejo Vicente M, Izquierdo Caballero R, Samaniego Fernández CM, Marín Urueña SI, Infante López ME, et al. Observación clínica de recién nacidos con factores de riesgo infeccioso, una práctica segura. An Pediatr (Barc). 2018;88:239–245.
Previous presentation: This study has been presented at the XXIII Congreso de Neonatología y Medicina Perinatal of the Sociedad Española de Neonatología, October 5–7, 2011; Oviedo, Spain; the XXIV Memorial Guillermo Arce y Ernesto Sanchez-Villares, November 25–26, 2011; Oviedo, Spain, and the XXIV Congreso de Neonatología y Medicina Perinatal and IV Congreso de Enfermería Neonatal; October 2–4, 2013; Barcelona, Spain.