Systematic childhood vaccination against meningococcus C has had a considerable impact on meningococcal invasive disease (MID). The aim of this study is to perform an analysis on the epidemiology, the clinical features, and the factors associated with a worse prognosis of MID, in the era of a meningococcal C vaccine.
Material and methodsThe study included confirmed cases of MID in children less than 15 years of age in Navarra, Spain, between 2008 and 2014. The risk of death or permanent sequelae was evaluated according to the presence of clinical features and analytical parameters at diagnosis.
ResultsThe average annual incidence was 7.9 cases per 100000 children, with the highest attack rate in children <1 year. Of 53 cases analysed, 87% were due to meningococcus B. Fever (100%), rash (91%), and elevation of procalcitonin (94%) were the most frequent findings at diagnosis. Some sign of shock was observed in 70% upon arrival at the hospital. The case-fatality rate was 3.8% and 10% survived with permanent sequelae. Glasgow coma scale <15 (odds ratio [OR]=9.2), seizure (OR=8.3), sepsis without meningitis (OR=9.1), thrombocytopenia (OR=30.5), and disseminated intravascular coagulation (OR=10.9) showed a greater association with a worse prognosis.
ConclusionThe MID continues to be a significant cause of morbidity and mortality in children. Therefore, new advances are needed in the prevention, early diagnosis, and detection of the factors associated with poor prognosis.
La vacunación sistemática infantil frente al meningococo C ha tenido un impacto considerable en la enfermedad meningocócica invasiva (EMI). El objetivo de este estudio es analizar la epidemiología, las manifestaciones clínicas y los factores asociados a un peor pronóstico de la EMI en la era de la vacuna antimeningocócica C.
Material y métodosSe analizaron los casos de EMI confirmados en menores de 15 años diagnosticados en Navarra entre 2008 y 2014, y se evaluó el riesgo de muerte o secuelas permanentes, según la presencia de determinados hallazgos clínicos o analíticos al diagnóstico.
ResultadosLa incidencia media anual fue 7,9 casos por 100.000 niños, con mayor tasa de ataque en niños < 1 año. De 53 casos analizados, el 87% fueron por meningococo B. Fiebre (100%), exantema (91%) y elevación de la procalcitonina (94%) fueron los hallazgos más frecuentes al diagnóstico. El 70% de los casos presentaba algún signo de shock a su llegada al hospital. La letalidad fue del 3,8% y un 10% sobrevivió con secuelas permanentes. Una puntuación en la escala de coma de Glasgow < 15 (odds ratio [OR]=9,2), convulsión (OR=8,3), sepsis sin meningitis (OR=9,1), trombocitopenia (OR=30,5) y coagulación intravascular diseminada (OR=10,9) se asociaron con un peor pronóstico.
ConclusiónLa EMI continúa causando una morbimortalidad importante en la población infantil, por lo que sigue siendo necesario avanzar en su prevención, en su diagnóstico temprano y en reconocer los factores asociados a mal pronóstico.
Invasive meningococcal disease (IMD), caused by the bacterium Neisseria meningitidis (N. meningitidis, known as meningococcus), is a severe infection that primarily affects young children.1–4
In Spain, the inclusion of the conjugate vaccine against group C meningococcus (MenC) in the routine immunisation schedule in 2001 was followed by a decline in the incidence of IMD, which was down to approximately 0.8 cases per 100000 inhabitants per year by 2012–2013.5 However, compared to other transmissible infectious diseases, it still is a considerable social, health care and economic burden and remains a top health priority.6,7
Invasive meningococcal disease typically manifests with acute sepsis and/or meningitis that may result in death within a few hours.2 In Spain, despite adequate treatment, its associated mortality is 10%, and 10%–20% of survivors experience sequelae.8,9
Invasive meningococcal disease poses a challenge to clinicians due to the difficulty of early diagnosis in some patients and to the complexity of the care required by the most severe cases. The initial signs and symptoms are often nonspecific, especially in infants,10 which complicates differential diagnosis.11,12 Early administration of antibiotics and initiation of life support are the cornerstones of treatment.7,13,14 In IMD, early identification of patients at risk of having a poor outcome from the first contact with primary care is of vital importance,12 as early treatment and transfer to intensive care is associated with increased survival and a decreased incidence of sequelae.15 To this end, several predictors of severity have been proposed.16
The aim of our study was to explore the epidemiology and clinical characteristics of IMD in the paediatric population of Navarra in the age of universal vaccination against meningococcus C, and to identify the clinical and laboratory findings at diagnosis that are associated with a poor prognosis.
Materials and methodsWe conducted a retrospective study between 2008 and 2014, a period in which the routine immunisation against meningococcus C was well established that we considered to be long enough to reflect the current situation of IMD. The population under study included every child aged less than 15 years residing in Navarra, which amounted to approximately 95500 inhabitants. We defined a case as any patient aged less than 15 years with clinical manifestations compatible with IMD and with microbiological confirmation of N. meningitidis in blood or cerebrospinal fluid (CSF) samples (isolation in culture, DNA detection by polymerase chain reaction, or antigen detection by immunoassay).
We obtained the epidemiological and microbiological data of all cases from the active IMD surveillance system records included in the Register of Notifiable Diseases of Navarra. We completed this information with data from medical records in 46 cases.
We calculated the incidence of IMD using the population size recorded in the census on January 1 for each age group and year as the denominator, using data from the Instituto Nacional de Estadística (National Institute of Statistics).
We defined sepsis, septic shock, severe sepsis and organ dysfunction based on the criteria of the 2005 International Consensus Conference on Pediatric Sepsis.17 We classified patients that did not meet the criteria for sepsis but in whom viable bacteria were isolated from blood as cases of bacteraemia. We took into account clinical signs of shock following the Spanish Clinical Practice Guideline on the Management of Invasive Meningococcal Disease7 (unusual skin colour, peripheral coldness, capillary refill time >2s, mental confusion or decreased level of consciousness, tachycardia or hypotension, respiratory difficulty or pain in the extremities). We defined disseminated intravascular coagulation (DIC) as the presence of laboratory findings characteristic of this pathology.18
We defined poor outcome as the presence of permanent sequelae (present 3 months after discharge) or death. The factors analysed for a potential association with poor outcome were: duration of symptoms before arrival to hospital (<12; 12–23 and ≥24h), manifestations of sepsis in the absence of meningitis, skin lesions either extensive (generalised or disseminated, found in the trunk and extremities) or advanced (purpura or ecchymosis), a score of less than 15 in the Glasgow coma scale, one or more episodes of seizures, clinical criteria of severe sepsis, leukopaenia (leucocytes <4500/mm3), neutropaenia (neutrophils <900/mm3), thrombocytopaenia (platelets <150000/mm3), product of platelet and neutrophil counts (PN product) of less than 40×109/L, base excess (BE) of less than −8, procalcitonin (PCT) greater than 100ng/mL, and DIC.
All the cases received inpatient care in the public health care network of Navarra, which consists of one tertiary referral hospital and two regional hospitals. To assess prognostic factors, we analysed the values recorded at the time of admission in any of the hospitals (initial history-taking, physical examination and blood work), except for DIC, for which we took into account the presence of clinical criteria at any point during the episode of disease.
To analyse the factors associated with a poor prognosis, we conducted a bivariate analysis comparing each clinical or laboratory finding (independent variable) and poor outcome (dependent variable). This analysis included the 46 cases for which we had access to medical records. We performed the comparisons with Fisher's exact test. We defined statistical significance as a p-value of less than .05.
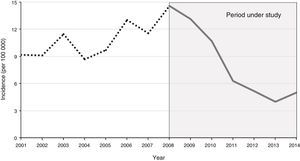
ResultsEpidemiologyThe study included a total of 53 cases of IMD between 2008 and 2014. The incidence of IMD showed a predominantly decreasing trend (Fig. 1). The mean annual incidence was 7.9 cases per 100000 individuals. Cases occurred predominantly in the winter months (weeks 1–4 and 45–53). We found no statistically significant differences between sexes (58% male, 42% female). The mean age of the patients was 2.7 years (standard deviation [SD], 4.5 years), and 83% of the children were aged less than 5 years. The annual incidence was highest in children aged less than one year (40.7 cases per 100000), followed by children aged 1–4 years (13.3 cases per 100000) and children aged 5–14 years (2.1 cases per 100000). In 87% of patients, the isolate was of the B serogroup, in 4% of the C serogroup and in 2% of the Y serogroup. Eight percent of cases were caused by nontypeable meningococci. Ninety-three percent of patients with IMD had been vaccinated with the MenC vaccine. Of the two cases with group C meningococcus, one was due to vaccination failure (the patient was correctly vaccinated for age) and the other occurred in a child whose parents had refused vaccination.
Clinical and laboratory findings at diagnosisThe most frequent clinical presentation was combined sepsis–meningitis (36%), followed by sepsis (34%), meningitis (17%) and bacteraemia (13%).
The mean time elapsed between the onset of symptoms and seeking care was 23±6.3h. Twenty-two percent sought care in the first 12h, forty-eight percent 12–23h after onset, and thirty percent at 24h or more. Eighty-one percent of patients presenting with sepsis or sepsis–meningitis sought care in the first 24h compared to forty-three percent of the patients that presented with meningitis or bacteraemia (p=.026).
The signs and symptoms present most frequently at diagnosis were fever, exanthem (haemorrhagic in most cases) and signs of shock (Table 1). Meningeal signs were negative in 48% of the patients in which meningitis or sepsis–meningitis was subsequently confirmed.
Clinical and laboratory findings at diagnosis in patients with invasive meningococcal disease aged less than 15 years in Navarra, 2008–2014.
| Clinical manifestations | Total N | Frequency N (%) |
|---|---|---|
| Axillary temperature ≥38°C | 46 | 46 (100) |
| Axillary temperature ≥39°C | 46 | 35 (76) |
| Exanthem | 46 | 42 (91) |
| Haemorrhagic exanthem | 46 | 33 (72) |
| Signs of shock | 46 | 32 (70) |
| Glasgow Coma Scale score <15 | 46 | 21 (46) |
| Seizure | 46 | 6 (13) |
| Blood test values | ||
| Procalcitonin >0.5ng/mL | 32 | 30 (94) |
| Leucocytes >10000/mm3 | 45 | 37 (82) |
| Base excess <−2 | 20 | 14 (70) |
| Polymorphonuclear leukocytes >70% | 45 | 27 (60) |
| C-reactive protein >10mg/dL | 45 | 25 (53) |
| Platelets <150000/mm3 | 45 | 5 (11) |
| Leucocytes <4500/mm3 | 45 | 4 (9) |
| Neutrophils <900/mm3 | 45 | 3 (7) |
One patient died on admission to hospital, so no laboratory data were available.
Procalcitonin level measurement started in year 2009.
The complete blood count detected leukocytosis in 82% of patients, neutrophilia in 60% and thrombocytopaenia in 11%. Procalcitonin was elevated in 94% of patients in whom it was measured, and C reactive protein was elevated in 53%. Measurement of pH detected metabolic acidosis in 70% of cases (Table 1).
Treatment and outcomeAll patients with IMD were admitted to the inpatient paediatric ward and 68% were also admitted to the paediatric intensive care unit (PICU) (100% of sepsis–meningitis, 67% of sepsis and 56% of meningitis cases). The mean total length of stay was 12±9.8 days, while the mean stay in the PICU was 6±7.2 days.
The most frequent type of organ dysfunction was cardiovascular (60%), followed by haematological (58%), respiratory (42%), neurologic (36%) and renal (9%). No patient developed liver dysfunction. Thirteen percent of patients developed DIC and one third had multiple organ dysfunction.
All the patients received intravenous antibiotherapy (98% cefotaxime and 2% ceftriaxone). Some patients also required volume expanders (69%), vasoactive and inotropic agents (67%), oxygen therapy (65%), corticosteroids (42%), intubation (10%) or transfusion of blood products (9%).
Condition at dischargeTwo patients aged 10 and 12 months died, resulting in a mortality of 3.8% and a mean annual mortality of 0.3 deaths per 100000 individuals (2.1 deaths per 100000 children <2 years). The two deaths occurred in patients with multiple organ failure secondary to septic shock that did not respond to treatment. The isolated strain was from serogroup B in one of these patients and nontypeable in the other. In both cases, death occurred within 12h from the onset of symptoms.
Of the 51 patients that survived, 24% experienced some sort of sequela (sepsis group, 39%; sepsis–meningitis group, 32%; and meningitis group, 11%), and the sequelae were permanent in 10%. The most frequent temporary sequelae were due to soft tissue necrosis. The permanent sequelae were deafness (40%), limb amputation (40%) and other neurologic impairment (20%).
Signs associated with poor outcomeA score of less than 15 in the Glasgow Coma Scale (odds ratio [OR]=9.2), having at least one seizure (OR=8.3), clinical manifestations of sepsis in the absence of meningitis (OR=9.1), thrombocytopaenia (OR=30.5) and DIC (OR=10.9) were significantly associated with poor outcome (Tables 2 and 3). The association for extensive or advanced skin lesions did not reach statistical significance.
Clinical factors associated with poor outcomes in patients with invasive meningococcal disease aged less than 15 years in Navarra, 2008–2014.
| Clinical factors | Total N | Poor outcome N (%) | Crude odds ratio (95% confidence interval) | pa |
|---|---|---|---|---|
| Sepsis without meningitis | ||||
| No | 33 | 2 (6.1) | 1 | |
| Yes | 13 | 5 (38.5) | 9.1 (1.5–78.2) | .015 |
| Severe sepsis | ||||
| No | 17 | 1 (5.9) | 1 | |
| Yes | 29 | 6 (20.7) | 4.1 (0.5–102.3) | .209 |
| Extensive/advanced skin lesions | ||||
| No | 23 | 1 (4.3) | 1 | |
| Yes | 23 | 6 (26.1) | 7.5 (1.0–187.2) | .053 |
| Glasgow Coma Scale score <15 | ||||
| No | 25 | 1 (4.0) | 1 | |
| Yes | 21 | 6 (28.6) | 9.2 (1.2–230.6) | .030 |
| Time elapsed between onset of symptoms to seeking care | ||||
| <12h | 10 | 1 (10.0) | 1 | |
| 12–23h | 22 | 4 (18.2) | 2.0 (0.2–4.6) | 1.000 |
| ≥24h | 14 | 2 (14.3) | 1.5 (0.1–19.2) | 1.000 |
| Seizures | ||||
| No | 40 | 4 (10.0) | 1 | |
| Yes | 6 | 3 (50.0) | 8.3 (1.1–65.3) | .040 |
Laboratory findings associated with poor outcome in patients with invasive meningococcal disease aged less than 15 years in Navarra, 2008–2014.
| Laboratory parameters (tested in blood, except the last) | Total N | Poor outcome N (%) | Crude odds ratio (95% confidence interval) | pa |
|---|---|---|---|---|
| Leucocytes <4500/mm3 | ||||
| No | 41 | 5 (12.2) | 1 | |
| Yes | 4 | 1 (25.0) | 2.3 (0.1–27.0) | .528 |
| Neutrophils <900/mm3 | ||||
| No | 42 | 5 (11.9) | 1 | |
| Yes | 3 | 1 (33.3) | 3.6 (0.1–54.4) | .399 |
| Platelets <150000/mm3 | ||||
| No | 39 | 2 (5.1) | 1 | |
| Yes | 6 | 4 (66.7) | 30.5 (3.6–377.1) | .001 |
| Procalcitonin >100ng/mL | ||||
| No | 27 | 1 (3.7) | 1 | |
| Yes | 5 | 2 (40.0) | 14.7 (0.9–526.1) | .068 |
| Disseminated intravascular coagulation | ||||
| No | 39 | 3 (7.7) | 1 | |
| Yes | 6 | 3 (50.0) | 10.9 (1.4–94.7) | .025 |
| Base excess <–8 | ||||
| No | 16 | 1 (6.2) | 1 | |
| Yes | 4 | 1 (25.0) | 4.5 (0.1–206.4) | .400 |
| Platelet * Neutrophil product <40×109/L | ||||
| No | 44 | 5 (11.4) | 1 | |
| Yes | 1 | 1 (100.0) | Not available | .133 |
| Leucocytesin cerebrospinal fluid <10/mm3 | ||||
| No | 38 | 4 (10.5) | 1 | |
| Yes | 2 | 0 (0.0) | 0 (0.0–34.3) | .808 |
We found a decreasing incidence of IMD in Navarra between 2008 and 2014, as has been documented for the rest of Spain.4 The increased incidence in winter3 and the greater burden of disease in young children are consistent with the literature.1,2 As is the case in other Western countries, we found a significant predominance of serogroup B.19 The low incidence of cases caused by serogroup B is associated with the high vaccination coverage with the MenC vaccine in Spain.20
The most frequent clinical presentation was sepsis, but in the literature there is variation in the most predominant form—sepsis or meningitis—from study to study.11,21 Patients presenting with sepsis and sepsis–meningitis sought care before patients with other forms of disease, probably on account of the rapid progression and severity of these cases. Fever and exanthem, which are symptoms frequently described in cases of IMD,15,21,22 were the manifestations found most frequently at the time of diagnosis. The exanthem was not haemorrhagic in two out of ten patients, which is a relevant finding,7 as less serious infections in children often present with non-haemorrhagic exanthem. Consistent with other studies,10 a considerable proportion of patients presented with some sign of shock on arrival to the hospital. This fact is worth noting, as some of these signs manifest in the early stages7 and knowing how to recognise them is important for the purpose of early diagnosis.11,12 Meningeal signs were negative in almost one out of every two patients presenting with sepsis–meningitis or meningitis, possibly because they manifest at a later time or because they often do not develop in infants.7,11
The abnormal laboratory findings were consistent with the previous literature.15,23 The proportion of patients with elevated PCT was higher than the proportion with elevated C-reactive protein. This may be related to the rapid progression of most cases and supports the hypothesis that due to its earlier elevation,24 PCT is more useful in the diagnosis of meningococcal disease.25
In IMD, there is a massive activation of the inflammatory cascade and the coagulation pathway, which causes damage to the internal organs of the patients.2 The severity of this disease is evinced by the percentage of patients admitted to the PICU and the need for life support measures. Our findings are consistent with those of other authors as to lethality, mortality and mean length of stay,6 with most deaths occurring in the first 24h,11,21 and the frequency and type of sequelae.8,9
Many studies have analysed clinical and laboratory markers, alone or in the form of scales, as potential prognostic factors in IMD.16,26–32 In our study, sepsis without meningitis, seizure, and a score of less than 15 in the Glasgow Coma Scale were the clinical factors with the highest predictive value for a poor outcome. We did not find an association between the duration of symptoms and poor outcome, probably because the most severe cases tend to seek care earlier and counteract a worse initial prognosis with earlier initiation of care. The association with the presence of extensive or advanced exanthem was not statistically significant, but is nevertheless considered relevant in prognostication.7,30,33 Thrombocytopaenia and DIC were the laboratory parameters that best predicted a poor outcome. This is a relevant finding, as the platelet count is an accessible and economic test with a demonstrated predictive value27 that is used in various composite scales, such as the PN product.29
Protocols for the management of paediatric patients with IMD can incorporate thrombocytopaenia as a marker for poor prognosis, one that could be particularly useful in facilities in which more sophisticated tests for other markers are not available. Children with thrombocytopaenia or other abnormal laboratory findings compatible with DIC, as well as children presenting with sepsis but not meningitis, a score of less than 15 in the Glasgow Coma Scale or that experience seizures should be considered at high risk.
The findings of this study must be interpreted taking its limitations into account. Although our study included all cases of IMD in Navarra, the sample size was small and restricted to a specific geographical area. The retrospective design may have led to biases. We did not consider whether patients with IMD had previously visited their primary care clinic or collect data for other symptoms such as headache, vomiting or photophobia.15,21 We were unable to obtain the laboratory values for all the patients. In some cases that resulted in permanent sequelae or death, the PCT, BE and leukocytecountin CSF were not available, so the association of these parameters with a poor outcome may be stronger than found in our analysis. Furthermore, in most cases, BE was measured in samples of venous blood, and the presence of DIC, which was documented at more advanced stages, may have been given more weight than other factors. We did not analyse psychological sequelae.
The recent introduction in the market of the meningococcal B vaccine in Spain marks the dawn of a new era in the prevention of IMD. This new development will probably lead to changes in this disease in the near future, so the situation that we have described may serve as the starting point for new studies evaluating the impact of the new vaccine in coming years.
To conclude, despite the significant advances made in the prevention of IMD with the routine immunisation of children against serogroup C meningococcus, the disease continues to be a significant health problem in the paediatric age group. The early recognition of symptoms, the optimisation of initial treatment in emergency care settings and the urgent transfer of patients to facilities with a PICU are key strategies to reducing mortality and the incidence of sequelae in children with IMD.
Conflict of interestsThe authors have no conflict of interests to declare.
We thank Dr Federico Martinón-Torres (and his colleagues), co-coordinator of the ESIGEM study, for his willingness to collaborate with us on this study.
Please cite this article as: Morales D, Moreno L, Herranz M, Bernaola E, Martínez-Baz I, Castilla J. Enfermedad meningocócica invasiva en Navarra en la era de la vacuna conjugada antimeningocócica C. An Pediatr (Barc). 2017;86:213–219.