Acute kidney injury (AKI) is a frequent complication of major surgery in newborn infants, especially those with congenital heart defects, which are associate with an increased morbidity and mortality and incidence of chronic kidney disease (CKD).1 There is little evidence on AKI in association with neonatal non-cardiac surgery (NNCS), for which the incidence has been estimated at 30%.2
To analyse the incidence of AKI in the context of NNCS and potential factors associated with its development and its impact on patient outcomes, we carried out a retrospective and cross-sectional study including patients who underwent NNCS in our hospital between 2014 and 2019, excluding those with pre-existing kidney failure or who developed AKI more than 7 days post surgery. We defined AKI and classified it into 3 stages applying the neonatal modified Kidney Disease Improving Global Outcome (KDIGO) criteria.3 We established two groups (AKI and no AKI) and compared them in terms of their demographic, clinical and laboratory characteristics and their outcomes. We performed a multivariate analysis adjusted for gestational age (GA) and birth weight (BW) as confounders to study factors associated with the development of AKI and to assess whether AKI could be a risk factor for mortality. We analysed the association of different variables with the duration of AKI with the Spearman correlation coefficient and by means of multiple linear regression adjusted for GA and BW. The statistical analyses were performed with the software STATA version 16.0 (Stata Corp, College Station, Texas, USA), and we considered P values of less than 0.05 statistically significant.
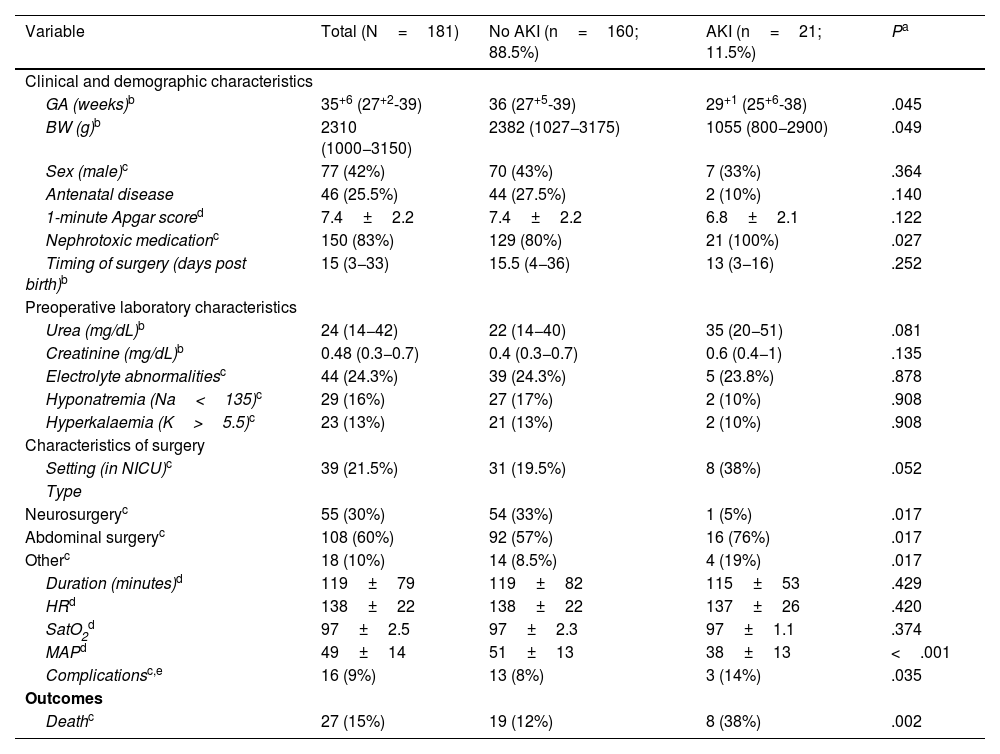
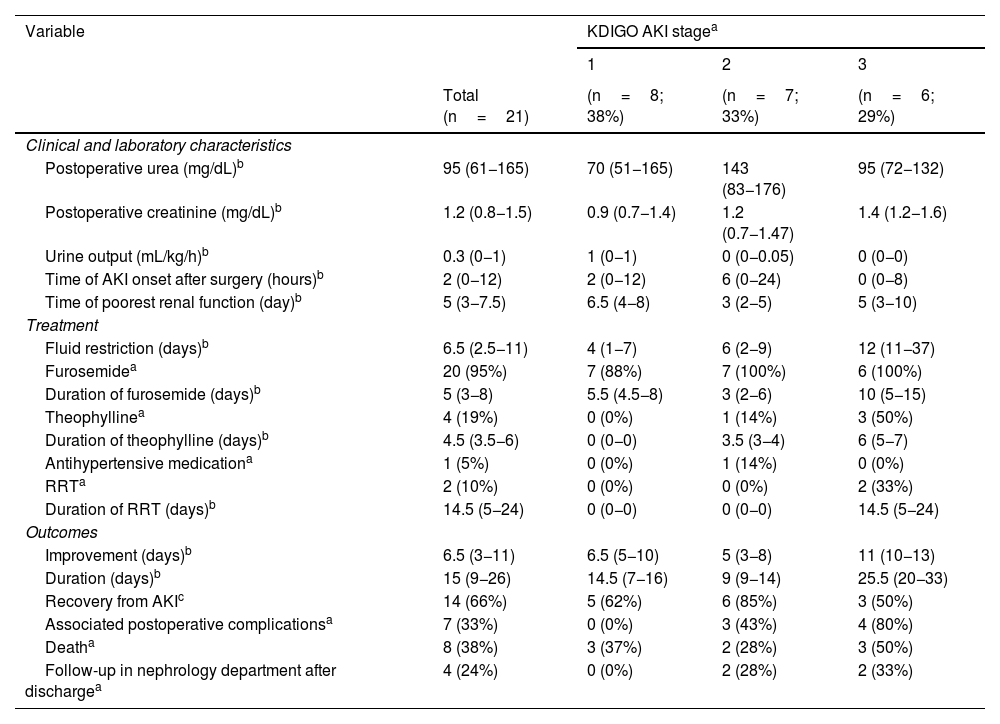
A total of 21/181 patients (11.6%) developed AKI. Table 1 presents the characteristics of the overall sample and the results of the univariate analysis comparing both groups, and Table 2 presents the characteristics of patients with AKI based on the KDIGO stage. Abdominal surgery (OR, 12.36; 95% CI, 1.6–96.8;=.017) and a lower intraoperative mean arterial pressure (MAP) (OR, 0.91; 95% CI, 0.86–0.97; P=.007) were associated with an increased incidence of AKI in the analysis adjusted for GA and BW. All patients with AKI received nephrotoxic drugs and 20/21 (95%) had received furosemide in continuous intravenous infusion at a mean dose of 2mg/kg/h.1,2 In addition, AKI was associated with an increase in mortality (OR, 4; 95% CI, 1.2–12; P=.017) adjusted for GA and BW. The main causes of death were refractory shock and multiple organ failure. In 14/21 of patients (66.6%), including all survivors at discharge and 1 deceased patient, there was evidence of recovery from AKI with normalization of creatinine levels. Increased duration of AKI was associated with longer duration of treatment with furosemide (ρ=0.50; P=.036) and a lower intraoperative MAP (ρ=0.55; P=.030), although this association did not persist in the multivariate analysis. Of the 13 survivors with AKI, only 4 (30%) were referred to the outpatient paediatric nephrology department for post-discharge follow-up: two who had stage 2 AKI, and two who had stage 3 AKI.
Descriptive analysis of the sample and comparison based on the development of acute kidney injury after surgery.
| Variable | Total (N=181) | No AKI (n=160; 88.5%) | AKI (n=21; 11.5%) | Pa |
|---|---|---|---|---|
| Clinical and demographic characteristics | ||||
| GA (weeks)b | 35+6 (27+2-39) | 36 (27+5-39) | 29+1 (25+6-38) | .045 |
| BW (g)b | 2310 (1000−3150) | 2382 (1027−3175) | 1055 (800−2900) | .049 |
| Sex (male)c | 77 (42%) | 70 (43%) | 7 (33%) | .364 |
| Antenatal disease | 46 (25.5%) | 44 (27.5%) | 2 (10%) | .140 |
| 1-minute Apgar scored | 7.4±2.2 | 7.4±2.2 | 6.8±2.1 | .122 |
| Nephrotoxic medicationc | 150 (83%) | 129 (80%) | 21 (100%) | .027 |
| Timing of surgery (days post birth)b | 15 (3−33) | 15.5 (4−36) | 13 (3−16) | .252 |
| Preoperative laboratory characteristics | ||||
| Urea (mg/dL)b | 24 (14−42) | 22 (14−40) | 35 (20−51) | .081 |
| Creatinine (mg/dL)b | 0.48 (0.3−0.7) | 0.4 (0.3−0.7) | 0.6 (0.4−1) | .135 |
| Electrolyte abnormalitiesc | 44 (24.3%) | 39 (24.3%) | 5 (23.8%) | .878 |
| Hyponatremia (Na<135)c | 29 (16%) | 27 (17%) | 2 (10%) | .908 |
| Hyperkalaemia (K>5.5)c | 23 (13%) | 21 (13%) | 2 (10%) | .908 |
| Characteristics of surgery | ||||
| Setting (in NICU)c | 39 (21.5%) | 31 (19.5%) | 8 (38%) | .052 |
| Type | ||||
| Neurosurgeryc | 55 (30%) | 54 (33%) | 1 (5%) | .017 |
| Abdominal surgeryc | 108 (60%) | 92 (57%) | 16 (76%) | .017 |
| Otherc | 18 (10%) | 14 (8.5%) | 4 (19%) | .017 |
| Duration (minutes)d | 119±79 | 119±82 | 115±53 | .429 |
| HRd | 138±22 | 138±22 | 137±26 | .420 |
| SatO2d | 97±2.5 | 97±2.3 | 97±1.1 | .374 |
| MAPd | 49±14 | 51±13 | 38±13 | <.001 |
| Complicationsc,e | 16 (9%) | 13 (8%) | 3 (14%) | .035 |
| Outcomes | ||||
| Deathc | 27 (15%) | 19 (12%) | 8 (38%) | .002 |
BW, birth weight; GA, gestational age; HR, heart rate; MAP, mean arterial pressure; NICU, neonatal intensive care unit; SatO2, oxygen saturation.
Characteristics of patients with acute kidney injury after surgery.
| Variable | KDIGO AKI stagea | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Total (n=21) | (n=8; 38%) | (n=7; 33%) | (n=6; 29%) | |
| Clinical and laboratory characteristics | ||||
| Postoperative urea (mg/dL)b | 95 (61−165) | 70 (51−165) | 143 (83−176) | 95 (72−132) |
| Postoperative creatinine (mg/dL)b | 1.2 (0.8−1.5) | 0.9 (0.7−1.4) | 1.2 (0.7−1.47) | 1.4 (1.2−1.6) |
| Urine output (mL/kg/h)b | 0.3 (0−1) | 1 (0−1) | 0 (0−0.05) | 0 (0−0) |
| Time of AKI onset after surgery (hours)b | 2 (0−12) | 2 (0−12) | 6 (0−24) | 0 (0−8) |
| Time of poorest renal function (day)b | 5 (3−7.5) | 6.5 (4−8) | 3 (2−5) | 5 (3−10) |
| Treatment | ||||
| Fluid restriction (days)b | 6.5 (2.5−11) | 4 (1−7) | 6 (2−9) | 12 (11−37) |
| Furosemidea | 20 (95%) | 7 (88%) | 7 (100%) | 6 (100%) |
| Duration of furosemide (days)b | 5 (3−8) | 5.5 (4.5−8) | 3 (2−6) | 10 (5−15) |
| Theophyllinea | 4 (19%) | 0 (0%) | 1 (14%) | 3 (50%) |
| Duration of theophylline (days)b | 4.5 (3.5−6) | 0 (0−0) | 3.5 (3−4) | 6 (5−7) |
| Antihypertensive medicationa | 1 (5%) | 0 (0%) | 1 (14%) | 0 (0%) |
| RRTa | 2 (10%) | 0 (0%) | 0 (0%) | 2 (33%) |
| Duration of RRT (days)b | 14.5 (5−24) | 0 (0−0) | 0 (0−0) | 14.5 (5−24) |
| Outcomes | ||||
| Improvement (days)b | 6.5 (3−11) | 6.5 (5−10) | 5 (3−8) | 11 (10−13) |
| Duration (days)b | 15 (9−26) | 14.5 (7−16) | 9 (9−14) | 25.5 (20−33) |
| Recovery from AKIc | 14 (66%) | 5 (62%) | 6 (85%) | 3 (50%) |
| Associated postoperative complicationsa | 7 (33%) | 0 (0%) | 3 (43%) | 4 (80%) |
| Deatha | 8 (38%) | 3 (37%) | 2 (28%) | 3 (50%) |
| Follow-up in nephrology department after dischargea | 4 (24%) | 0 (0%) | 2 (28%) | 2 (33%) |
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; RRT, renal replacement therapy.
The development of AKI is a complication to be considered in NNCS due to its frequency and associated morbidity and mortality, as reflected by the results for the primary outcome in our study, a significantly increased mortality in neonates with AKI (38%) compared to those without AKI (12%). Mortality in infants with AKI was lower in the study conducted by Wu et al. (18%),2 which could be due to the exclusion of patients with characteristics that could be associated with greater severity. The factors associated with the development of AKI in the context of NNCS in our hospital, such as smaller GA, smaller BW, the use of nephrotoxic medication, abdominal surgery, a lower MAP during surgery and intraoperative complications, were consistent with the findings of previous studies and make sense from a pathophysiological standpoint.4,5 Therefore, patients with these risk factors would benefit from closer monitoring of renal function for early detection and treatment of AKI. Although the design of our study did not allow us to assess causality, the univariate association between longer duration of AKI and longer duration of treatment with furosemide found in our study, given the known nephrotoxicity of this drug, suggests the need to optimise the duration of treatment with furosemide in this clinical scenario. It is also worth noting that even if the creatinine level reverts to baseline, a follow-up by a paediatric nephrologist is recommended at 3 months to evaluate recovery from AKI and assess for hypertension and proteinuria in order to establish the risk of progression to CKD.6
The main limitations of the study were those intrinsic to its retrospective design and it having been conducted in a single centre, in addition to the lack of consensus regarding the monitoring of postoperative creatinine levels, on account of which some patients may not have been included in the sample if they maintained an adequate urine output. Due to the above, we think that prospective longitudinal studies in larger samples and with a standardised renal function protocol during the hospital stay and nephrological follow-up after discharge are required to determine the prognosis of these patients more accurately.
Ethical considerationsThe study adhered to the World Medical Association International Code of Ethics (Declaration of Helsinki).
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.
Previous meeting: the contents of this article were presented as an oral at the 2nd Online Congress of the Asociación Española de Pediatría, 2021.