Cystic fibrosis (CF) is a progressive multisystemic disease that impairs quality of life. Respiratory manifestations are the main source of morbidity and mortality, and chest physiotherapy (CPT) is one of the main approaches used to improve mucociliary clearance.1 Mechanical insufflation–exsufflation (MIE) devices deliver a high enough pressure to produce a shearing force that dislodges and mobilises secretions outward. There is evidence that MIE can improve airway clearance in patients with neuromuscular diseases2 and on its use for treating bronchiectasis in patients without CF,3 but further research is needed to support its use in children with CF.1
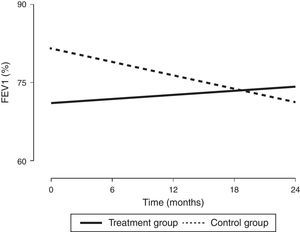
In order to assess the impact of MIE on lung function in children with a diagnosis of CF, we designed a prospective study with a 24-month followup to compare the combined use of CPT—which is part of the routine approach to CF management—and MIE (treatment group, n=9) with the use of CPT alone (control group, n=11). Every participant met all the inclusion criteria and none of the exclusion criteria (Table 1). All procedures were performed after obtaining the informed consent of the parents. The intervention in the treatment group was performed every 21 days for 45min. It consisted of three cycles of six to eight breaths with the patient in a reclined position (inspiratory time, 5s; expiratory time, 5s; pause time, 2s; pressure, +30 to −35cmH2O; device, Philips CoughAssist®). To better clear bronchial obstruction, we applied pressure in the abdomen. We performed pulse oximetry before and after each session. Adhering to the guidelines of the Sociedad Española de Neumología y Cirugía Torácica (Spanish Society of Pulmonology and Thoracic Surgery [SEPAR]), we assessed lung function at baseline and at 6, 12, 18 and 24 months of treatment by means of spirometry, defining improvement as an increase of at least 5% in the forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1); and worsening as a decrease of 5% or more (Table 1). Two of the nine patients in the treatment group were eventually excluded, one due to fear of the device and the other due to lack of cooperation. Although the baseline lung function of patients in the treatment group was worse than that of patients in the control group, we observed an increasing trend in FEV1 (%) after the intervention that was sustained through the followup (Fig. 1), as well as a statistically significant increase in FVC (%). In fact, the improvement in FEV1 (%) was positively and significantly correlated with receiving CPT (r=0.47; P<.05) and with improvement in FVC (%) (r=0.72; P<.05). In the control group, we observed statistically significant decreases in FEV1 (%) and FVC (%). Table 1 shows the clinical characteristics of the patients. The results of the Kaplan–Meier analysis of variations in percent FEV1 between groups were consistent with the improvement observed in the patients that received the treatment, as it estimated a six-month difference between both groups in favour of the treatment group (P<.05). As for nutritional status, we found no significant differences in body mass index between the groups, a parameter that has been assessed in the paediatric population with CF in recent years.4
Clinical characteristics of the patients.
| Patient numbera | Age/sex | Genotype GenA/GenB | Pancreatic sufficiency (yes/no) | Main presenting feature | Baseline BMI | BMI at 24 months | ΔFEV1/ΔFVC (%) | Disease progression |
|---|---|---|---|---|---|---|---|---|
| Patients treated with MIE | ||||||||
| 1 | 6.7/F | F508 | No | Pneumonia | 15.6 | 17.6 | −20.3/−10.2 | Worsening |
| 2 | 6.1/F | F508 | No | Faltering weight | 16.1 | 17.3 | 26.8/27.7 | Improvement |
| 3 | 6.6/M | F508/G542V | No | Meconium ileus | 16.1 | 16.4 | 4.5/10.9 | Stable |
| 4 | 14.3/F | R334W/R347P | Yes | Persistent cough | 18.8 | 19.5 | 5.4/5.9 | Improvement |
| 5 | 7.5/F | 2183AA-G/Q850X | No | Faltering weight | 16.3 | 17.6 | 18.9/20.2 | Improvement |
| 6 | 8.7/M | F508 | No | Faltering weight | 14.2 | 18.5 | 4.9/19.7 | Improvement |
| 7 | 11.5/F | 2183AA-GF508 | No | Persistent cough | 16.1 | 19.6 | 11.7/9.9 | Improvement |
| Patients not treated with MIE | ||||||||
| 8 | 6.8/F | F508 | No | Meconium ileus | 14.6 | 16.6 | −8.7/−6.2 | Worsening |
| 9 | 6.3/F | F508/I507 | No | Persistent cough | 15.4 | 16.8 | −5.8/−8 | Worsening |
| 10 | 11.1/F | F508 | No | Meconium ileus | 15.8 | 19.11 | −10.8/−9.2 | Worsening |
| 11 | 10.2/M | F508 | No | Faltering weight | 16.2 | 19.2 | −27.7/−27.4 | Worsening |
| 12 | 10.8/M | F508 | No | Meconium ileus | 15.8 | 17.9 | −22.8/−5 | Worsening |
| 13 | 7.2/M | F508 | No | Faltering weight | 15.9 | 17.7 | −4.2/−6.9 | Stable |
| 14 | 9.4/F | F508/2184 | Yes | Pancreatitis | 14.7 | 17.9 | −5.4/−4.1 | Stable |
| 15 | 11.7/M | W1282X-R334W | Yes | Persistent cough | 16.1 | 19.6 | −4.6/−0.8 | Stable |
| 16 | 9.1/M | W1282X-R334W | Yes | Persistent cough | 16.4 | 18.8 | −16.1/−16.7 | Worsening |
| 17 | 14.5/F | F508/3272-16AG | Yes | Persistent cough | 17.9 | 20.4 | −19.7/−26 | Worsening |
| 18 | 14.3/F | F508/I507 | No | Faltering weight | 18.2 | 18.9 | −15.2/−11.1 | Worsening |
BMI, body mass index (in kg/m2); F, female; FEV1, forced expiratory volume in the first second, expressed as percentage (%); FVC, forced vital capacity, expressed as percentage (%); M, male; MIE, mechanical insufflation–exsufflation; Δ, change in lung function parameter. FEV1 and FVC values were expressed as percentages of the predicted theoretical value for age, weight and height based on the Spanish reference population.
The patients included in the table met the following inclusion criteria, and none of the exclusion criteria. Inclusion criteria: diagnosis of CF based on internationally accepted criteria; undergoing periodic check-ups in the CF unit; age between 6 and 14 years; ability to perform manoeuvres required for lung function testing; understanding the purpose of the study; signed informed consent. Exclusion criteria: patients at radiologic or clinical risk of pneumothorax or pneumomediastinum; patients with barotrauma in the month prior to entry in the study; past history of massive or life-threatening haemoptysis; transplant recipients or patients awaiting a transplant.
Changes in lung function (expressed as %FEV1) from baseline to the end of the intervention. FEV1, forced expiratory volume in the first second expressed as percentage. The values were expressed as the percentage of the theoretical value for individuals of the same age, weight and height based on the Spanish reference population.
The clinical characteristics of our patients were consistent with those described in the literature,4 although the spirometric values in the treatment group were below those reported in previous studies,4,5 a circumstance that probably contributed to their decision to participate in the study. As Table 1 shows, while lung function did not improve in any of the patients in the control group after 24 months, in the treatment group it improved in five out of the seven patients, remained stable in one, and worsened only in one. The values of FEV1 (%) in the treatment group tended to improve with the intervention (Fig. 1), while the control group exhibited the natural course of CF, that is, a progressive decline in lung function.5 Based on these results, the proposed CPT intervention could slow down disease progression. In our study, we did not observe any side effects associated with the use of the device, and the technique was safe and well tolerated. We only found that coughing produced irritation in the pharynx or headache in some instances, and these effects were not at play in the two patients that dropped out. In conclusion, this is the first paediatric case series describing the combined use of conventional CPT and MIE in patients with CF, and it demonstrated an improvement in lung function over a follow-up period of at least two years. Consequently, this may be a good approach for preventing lung function impairment in this population, although multicentric studies need to be developed to reach solid evidence-based conclusions.
We thank the Asociación contra la Fibrosis Quística (Association against Cystic Fibrosis) of Malaga.
Please cite this article as: Fuentes LA, Caro P, Garcia-Ruiz AJ, Muñoz Gómez G, Martín-Montañez E. Mejora de la función pulmonar en fibrosis quística mediante insuflación-exuflación mecánica. An Pediatr (Barc). 2017;86:350–352.