Due to its ubiquity and its ability to survive in humid environments and to colonise the gastrointestinal and/or respiratory tracts, Serratia marcescens is responsible for multiple nosocomial outbreaks,1 especially in paediatric patients and specifically in newborns, who suffer the direst consequences.2,3 In some instances, it is possible to identify the source of infection, which is of particular relevance in outbreaks involving antiseptic solutions on account of their impact.3 In this letter, we describe our experience in a children's hospital in the context of a nationwide S. marcescens outbreak associated with the use of contaminated chlorhexidine (CHX) antiseptic solution4 that occurred between August 2014 and January 2015 and comprised 148 cases (86 confirmed) in 10 Autonomous regions. Although most of the affected patients were aged more than 65 years, 8 children were affected in our hospital, between November 22 and December 16, 2014. All developed bacteraemia and had favourable outcomes, except 1 infant who died within 24h from diagnosis (mortality of 12.5%).
Initially, when we were still unaware of the national scope of the outbreak, we hypothesised that there must be a common source/reservoir of S. marcescens, a fluid medium somewhere in the surgical suite of the hospital, where the first 5 affected patients had been treated. Since culture of the CHX-based antiseptic solutions in the surgical suite was negative and S. marcescens was isolated from a bag of packed red blood cells in a patient that had received a transfusion in the paediatric ICU and not undergone surgery, we considered transfusion another potential route of infection, as all the affected patients had received transfusions prior to the diagnosis of bacteraemia.
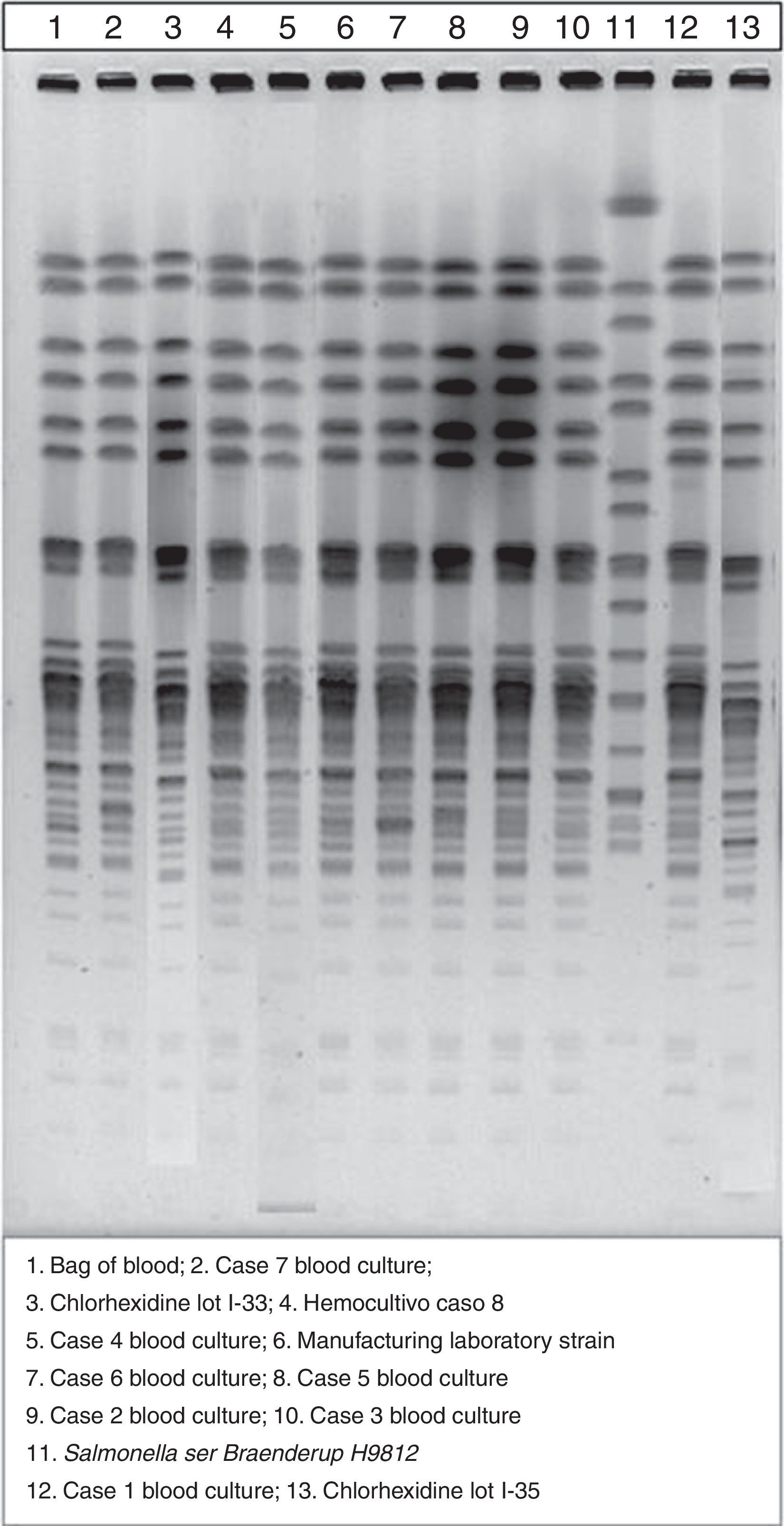
With the investigation underway, when 7 of the 8 cases had been detected, the Spanish Agency of Medicines and Medical Devices issued a recall of skin antiseptic based on CHX in aqueous or alcohol solution that had been distributed in 2014 (lots I-15, I-11, I-28, I-29, I-30, I-33 and I-35), while the Epidemiological Surveillance System of Andalusia (SVEA) of Andalusia warned us that the use of this antiseptic was probably the cause of the outbreak at our hospital. Having received this warning, we verified that the units affected by the outbreak had been using products from several of the lots included in the recall. Unlike other published studies regarding this outbreak,5 we performed pulsed-field gel electrophoresis for the molecular analysis of isolates from patient samples, those obtained from new samples of CHX solutions removed from use in the hospital (lots I-33 and I-35) and the strain provided by the laboratories that distributed the contaminated antiseptic, and found that all isolates were from a single clone (Fig. 1).
The procedures that most likely contributed to the transmission of infection were those whose performance was preceded by the use of this antiseptic, such as disinfection of the skin prior to surgery or catheterisation, and disinfection of 3-way stopcocks before transfusion. This was consistent with the information received in the outbreak report, where the medical procedure associated with the use of the CHX solution was catheterisation in 70% of cases.4
The rapid identification and notification of the outbreak, the implementation of preventive measures and molecular testing allowed the control of an outbreak affecting the paediatric age group, a particularly vulnerable population. We ought to highlight that our study evinced the clonal relationship of all isolates from the outbreak with the original contaminant strain, and that cases were restricted to the paediatric population, including one death that could be attributed to infection by S. marcescens out of the 148 patients affected nationwide. It is essential that we improve the communication between Public Health Agencies and health care facilities, as the problem started in 2014 and the outbreak persisted through December of the same year.4
We thank the Pediatric Critical Care and Emergency Unit, Department of Pediatrics and Department of Pediatric Surgery of the Pediatric University Hospital Virgen del Rocío, the Epidemiological Surveillance System of Andalusia and the Regional Blood transfusion center of Seville for their inestimable collaboration in managing the outbreak and their invaluable contributions.
Please cite this article as: Morillo Á, Torres MJ, Alonso Salas MT, Conde M, Aznar J. Implicación de un brote nacional de infección por Serratia marcescens asociado a clorhexidina contaminada en un hospital pediátrico. An Pediatr (Barc). 2018;88:171–172.