Juvenile recurrent chronic parotitis (JRCP) is a rare disease of unknown cause. There is a growing interest in its autoimmune aetiology and its relationship with dysfunctions of cellular and humoral immunity, although there is no agreed protocol for complementary investigations for its study. A consecutive series of cases is presented where the immune alterations and associated autoimmune disorders are investigated, proposing a study algorithm.
Patients and methodsA retrospective study was carried out on patients who had JRCP during the period from 2013 to 2016 and a follow-up of at least 2 years. After its clinical and ultrasound diagnosis, complementary examinations were systematically carried out to investigate infectious, immune, and autoimmune diseases.
ResultsOf a total of 36 patients with inclusion criteria, 16 (44%) were found with some analytical alteration of a non-specific immunological nature (positive ANA, high IgG, low complement factor 4), or associated with a specific diagnosis, as occurred in 11 patients: Selective IgA deficiency (2), Sjögren’s syndrome associated or not with systemic lupus erythematosus (3), coeliac disease associated or not with diabetes mellitus (4), Hashimoto’s thyroiditis (1), and acquired immunodeficiency syndrome (1).
ConclusionJuvenile recurrent chronic parotitis can be considered a sentinel sign of other diseases of immunological/autoimmune aetiology for which the diagnosis, monitoring and early treatment can improve its prognosis. Viral infectious aetiology, with the exception of HIV, is not a priority in the study of recurrences.
La parotiditis crónica recurrente juvenil (PCRJ) es una enfermedad infrecuente de causa desconocida. Existe un creciente interés por su etiología autoinmune y su relación con disfunciones de la inmunidad celular y humoral aunque no existe un protocolo consensuado de investigaciones complementarias para su estudio. Se presenta una serie consecutiva de casos donde se investigan las alteraciones inmunes y trastornos autoinmunes asociados, proponiendo un algoritmo de estudio.
Pacientes y métodosSe realizó un estudio retrospectivo de pacientes que presentaron PCRJ durante el periodo de 2013 a 2016 y seguimiento de al menos 2 años. Tras su diagnóstico clínico y ecográfico se realizaron de forma sistemática exámenes complementarios para investigación de patologías infecciosas, inmunes y autoinmunes asociadas.
ResultadosDe un total de 36 pacientes con criterios de inclusión, se encontraron 16 (44%) con alguna alteración analítica de carácter inmunológico inespecífico (ANA positivo, IgG elevada, factor 4 del complemento bajo) o asociada a un diagnóstico específico como ocurrió en 11 pacientes: déficit selectivo de IgA (2), síndrome de Sjögren asociado o no a lupus eritematoso sistémico (3), celiaquía asociada o no a Diabetes mellitus (4), tiroiditis de Hashimoto (1) y síndrome de inmunodeficiencia adquirida (1).
ConclusiónLa parotiditis crónica recurrente juvenile puede considerarse un signo centinela de otras enfermedades de etiología inmunológica/autoinmune cuyo diagnóstico, seguimiento y tratamiento precoz puede mejorar su pronóstico. La etiología infecciosa vírica, exceptuando el VIH, no es prioritaria en el estudio de recurrencias.
Juvenile recurrent parotitis (JRP) is defined as recurrent parotid inflammation of a non-obstructive and non-suppurative nature. It presents as a unilateral or bilateral swelling of the parotid gland with 2 or more episodes occurring before puberty. Episodes usually manifest with pain, redness and fever of less than 1 week’s duration that tend to resolve with symptomatic treatment and are separated by asymptomatic periods.1–4 Although the age at onset varies widely, ranging from 2 to 14 years, there are 2 incidence peaks at ages 2–5 years and age 10 years, and it is more prevalent in boys. It is a clinical diagnosis that is usually confirmed by sonographic findings indicative of sialectasis5 after ruling out other possible causes, such as ductal malformation, presence of sialoliths in Stenson’s duct, acute viral or bacterial parotitis, gland tumours and systemic allergic or autoimmune disorders.
Although JRP is the second leading cause of salivary gland disease following mumps, it is an infrequent condition and its aetiology, which is probably multifactorial, is not understood. Historically, chronic recurrent parotitis has been described in association with Sjögren syndrome (SS), hypogammaglobulinemia, IgG3 deficiency, IgA deficiency1 and as a frequent manifestation in patients with HIV infection.6 Its development may be part of an underlying immune disease, so there is a growing interest in potential underlying autoimmune mechanisms and their association with defects in cellular and humoral immunity, although standardised approach for the diagnostic workup of JRP has yet to be established.
We present a consecutive case series of patients with JRP frequently associated with immune disorders, especially SS, coeliac disease and IgA deficiency, which supports the autoimmune hypothesis. We also propose a protocol for the diagnostic workup of JRP based on our findings and a review of the current literature.
Sample and methodsThe aim of the study was to analyse the presence of serological abnormalities and the associated diagnoses in patients with JRP.
We conducted a retrospective study in patients referred to the outpatient clinics of a tertiary care hospital with a working diagnosis of JRP, defined as 2 or more episodes of symptomatic, non-obstructive and non-suppurative unilateral or bilateral parotitis in a child aged 1–14 years. We reviewed the patients managed consecutively between 2013 and 2016, both included, with a minimum duration of follow-up of 2 years post diagnosis. Parotid involvement was confirmed in all cases by compatible sonographic findings: an enlarged heterogeneous parotid gland, with unilateral or bilateral involvement, and areas of hypoechogenicity measuring 2–4 mm and/or hyperaemia on Doppler ultrasound indicative of sialectasis or lymphocytic infiltration.5 Magnetic resonance imaging (MRI) was not used for diagnosis in any case.
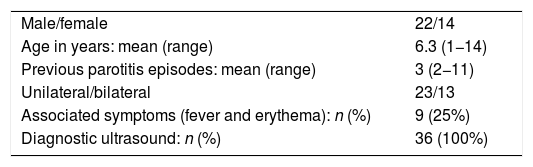
We collected data on basic clinical variables, such as the age at onset, sex, previous episodes of parotitis and whether the inflammation affected one or both glands to allow future comparison with other case series in the literature, which was not the objective of our study.
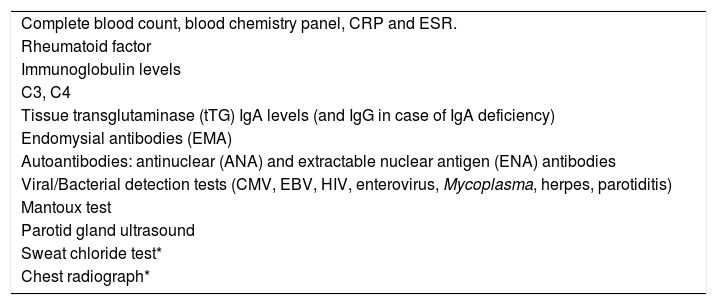
We collected data on serological tests results and the findings of other diagnostic tests performed per the established protocol, which was based on a case series published by Concheiro et al.1 and adapted to our setting (Table 1). In cases in which the results of the initial serological tests suggested associated disorders, the evaluation was completed with additional laboratory and imaging tests to confirm the diagnosis. We recorded the final diagnosis established in each case by the end of the follow-up period and considered patients in whom no other disease had been identified cases of isolated JRP.
Tests performed for evaluation of juvenile recurrent parotitis.
| Complete blood count, blood chemistry panel, CRP and ESR. |
| Rheumatoid factor |
| Immunoglobulin levels |
| C3, C4 |
| Tissue transglutaminase (tTG) IgA levels (and IgG in case of IgA deficiency) |
| Endomysial antibodies (EMA) |
| Autoantibodies: antinuclear (ANA) and extractable nuclear antigen (ENA) antibodies |
| Viral/Bacterial detection tests (CMV, EBV, HIV, enterovirus, Mycoplasma, herpes, parotiditis) |
| Mantoux test |
| Parotid gland ultrasound |
| Sweat chloride test* |
| Chest radiograph* |
Source: Concheiro et al.1.
We collected retrospective data on the results of viral and bacterial serological tests (cytomegalovirus, Epstein-Barr virus, enterovirus, parotiditis, adenovirus and Mycoplasma) conducted in the first episode of parotitis managed in the emergency department, performed based on the clinician’s judgment.
The descriptive analysis of the data, with calculation of percentages, mean values and ranges, was performed with the software Microsoft Excel version 14.0.
The study was approved by the Scientific and Ethics Research Committee of the Hospital La Fe (file no. 2019-303-1).
ResultsWe analysed a total of 36 cases that met the inclusion criteria. The duration of follow-up was at least 2 years, up to a maximum of 6 years, by the end of the study. Table 2 presents the main epidemiological characteristics of the patients.
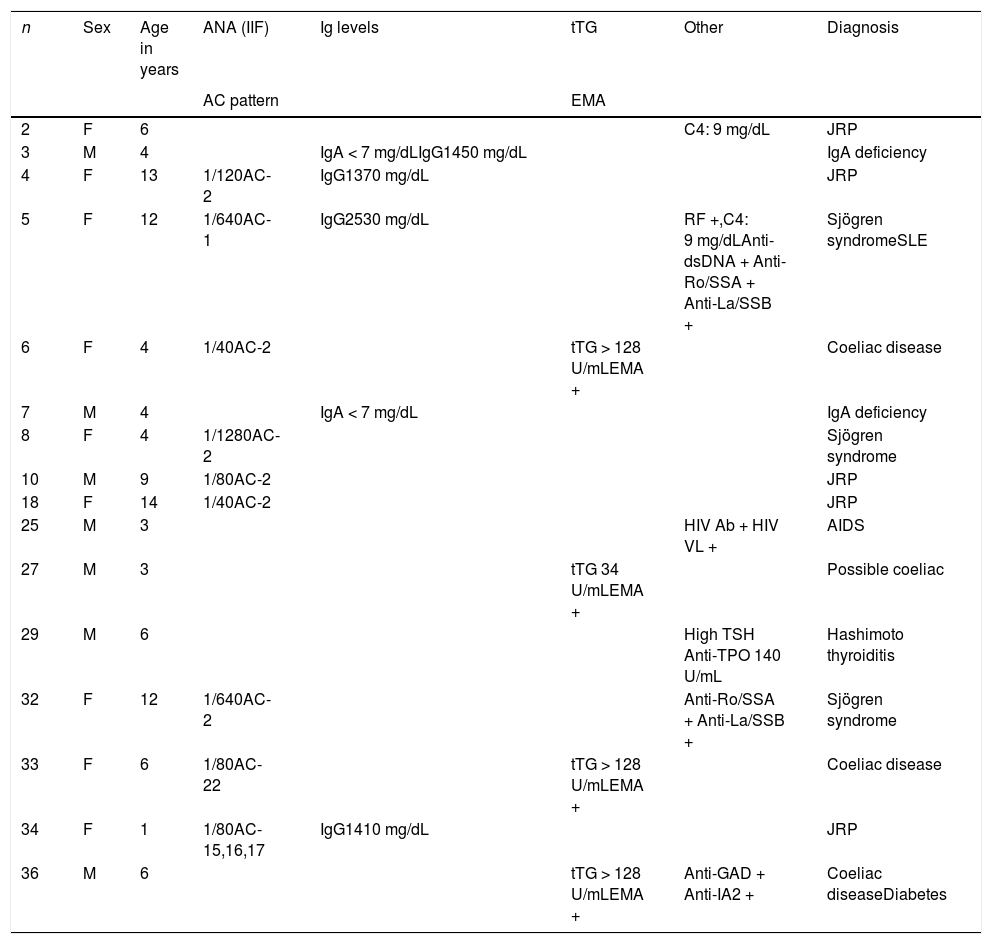
Serological abnormalities were detected in 16 of the 36 patients (44.4%), either nonspecific or, as occurred in 11, associated with a specific final diagnosis (Table 3). In the remaining 20 cases, the laboratory results were normal and there were no additional associated with JRP.
Positive results of serological tests and final diagnosis.
| n | Sex | Age in years | ANA (IIF) | Ig levels | tTG | Other | Diagnosis |
|---|---|---|---|---|---|---|---|
| AC pattern | EMA | ||||||
| 2 | F | 6 | C4: 9 mg/dL | JRP | |||
| 3 | M | 4 | IgA < 7 mg/dLIgG1450 mg/dL | IgA deficiency | |||
| 4 | F | 13 | 1/120AC-2 | IgG1370 mg/dL | JRP | ||
| 5 | F | 12 | 1/640AC-1 | IgG2530 mg/dL | RF +,C4: 9 mg/dLAnti-dsDNA + Anti-Ro/SSA + Anti-La/SSB + | Sjögren syndromeSLE | |
| 6 | F | 4 | 1/40AC-2 | tTG > 128 U/mLEMA + | Coeliac disease | ||
| 7 | M | 4 | IgA < 7 mg/dL | IgA deficiency | |||
| 8 | F | 4 | 1/1280AC-2 | Sjögren syndrome | |||
| 10 | M | 9 | 1/80AC-2 | JRP | |||
| 18 | F | 14 | 1/40AC-2 | JRP | |||
| 25 | M | 3 | HIV Ab + HIV VL + | AIDS | |||
| 27 | M | 3 | tTG 34 U/mLEMA + | Possible coeliac | |||
| 29 | M | 6 | High TSH Anti-TPO 140 U/mL | Hashimoto thyroiditis | |||
| 32 | F | 12 | 1/640AC-2 | Anti-Ro/SSA + Anti-La/SSB + | Sjögren syndrome | ||
| 33 | F | 6 | 1/80AC-22 | tTG > 128 U/mLEMA + | Coeliac disease | ||
| 34 | F | 1 | 1/80AC-15,16,17 | IgG1410 mg/dL | JRP | ||
| 36 | M | 6 | tTG > 128 U/mLEMA + | Anti-GAD + Anti-IA2 + | Coeliac diseaseDiabetes |
Ab, antibody; AC pattern, anti-cell pattern (https://www.ANApatterns.org); ANA (IIF), antinuclear antibody (indirect immunofluorescence); EMA, anti-endomysial antibody; GAD, glutamic acid decarboxylase autoantibodies; IA2, tyrosine phosphatase-related islet antigen 2; Ig, immunoglobulin; JRP, juvenile recurrent parotitis; RF, rheumatoid factor; SLE, systemic lupus erythematosus; TPO, thyroid peroxidase; TSH, thyroid-stimulating hormone; tTG, tissue transglutaminase antibodies; VL, viral load.
Low C4: complement component 4 < 10; low IgA: level < 6 mg/dL; high IgG: level > upper limit of normal for age.
In our retrospective review of records, we only found documentation for viral tests performed in the first parotitis episode for 4 patients, who had positive results for IgM and IgG antibodies against Mycoplasma pneumoniae, adenovirus, enterovirus and Epstein-Barr virus in one patient each. Starting from the second episode of JRP, viral tests were performed in every patient. There were no IgM-positive results. One patient tested positive for HIV with a detectable viral load and a final diagnosis of AIDS. In every case, tests for detection of mumps were negative for IgM antibodies and positive for IgG antibodies, confirming correct immunization in adherence with the routine vaccination schedule. In our retrospective review of records, we only found documentation for viral tests performed in the first parotitis episode for 4 patients, who had positive results for IgM and IgG antibodies against Mycoplasma pneumoniae, adenovirus, enterovirus and Epstein-Barr virus in one patient each.
None of the patients had a positive Mantoux test, and sweat tests and chest radiographs performed based on clinical suspicion were normal.
The confirmation of the diagnoses of diseases associated with JRP and their subsequent follow-up was performed by the applicable medical specialist based on current diagnostic guidelines in paediatric gastroenterology, rheumatology, infectious diseases and endocrinology. Patients with a final diagnosis of isolated JRP were followed up by the division of general paediatrics and at the primary care level; none of them required surgical intervention or intraductal infusion of drugs during the follow-up, and subsequent episodes were managed with conservative treatment.
DiscussionFrom a demographic standpoint, the patients in this case series did not differ from those in other published paediatric series.1–4 However, serological abnormalities and the final diagnosis of autoimmune disease stemming from the warning sign of JRP were clearly more frequent in our series, which consisted of patients included consecutively over a period of 4 years.
Although MRI has been described as a useful technique for diagnosis and follow-up,7 in our study diagnosis was based on the detection of pathological features in the parotid gland by means of high-resolution ultrasound, which is considered the first-line imaging modality on account of its sensitivity for the detection of abnormalities in the salivary glands.5 However, this selection criterion may be questioned by authors that consider that MRI offers more advantages for both diagnosis and follow-up.7
Once tuberculosis and cystic fibrosis are ruled out based on the current low prevalence of these diseases and negative results of the tuberculin skin test, sweat test and chest radiograph, and once acute viral diseases are ruled out by serological testing (except for HIV), the evaluation should focus on screening for immune and autoimmune disorders. We now proceed to discuss the results found in our sample in these areas.
Viral aetiologyThe positive result for HIV in the otherwise asymptomatic girl aged 3 years, who had been born in a different country after inadequate monitoring during pregnancy, combined with the positive HIV test in the mother, confirmed vertical transmission of HIV. At the time of diagnosis, the disease was in its early stages with a normal CD4 count and mild immunosuppression. It is known that HIV-associated salivary gland disease tends to develop in the early stages of infection, in some cases before deterioration of the immune system becomes apparent, which makes parotitis an important warning sign for suspicion of HIV. Its pathogenesis is unclear, and some authors have proposed an autoimmune or chronic inflammatory aetiology as opposed to a purely infectious one.6 It is more frequent in children compared to adults, and tends to follow a benign course, as was the case in our patient. When JRP is detected, it is important to include HIV in the differential diagnosis, especially in children from foreign countries with unknown maternal HIV status or born to mothers in which HIV testing or monitoring was only performd in the first trimester of pregnancy.
Testing for detection of non-HIV viruses performed in recurrent episodes of parotitis8 did not identify any acute infections, so it may not be a priority in the initial workup of JRP due to the lack of a temporal association with the development of these episodes. However, the positive IgM results for non-HIV viral infection obtained in the first episode of parotitis in 4 of our patients in whom the test was ordered based on the clinician’s judgment suggests that an initial viral infection could cause immune changes prolong the period during which patients experience recurrent inflammatory episodes until their spontaneous resolution in puberty.9
There is also evidence in adults of a potential association between SS-like sialadenitis and chronic infection by hepatitis C virus.10
IgA deficiencyThe detection in our series of 2 cases of selective IgA deficiency could be due to chance given the high documented prevalence of this disorder in the general population (1 case per 400–3000 donors). However, Fazekas et al.11 identified 5 cases in a total of 17 patients with JRP. Although the authors acknowledged that a clear association has yet to be established, they remarked on the growing interest in serological abnormalities in patients with JRP and the publication of individual case reports of patients with JRP and selective IgA deficiency. Other authors have since published case reports of patients with JRP and isolated IgA deficiency.12,13 In clinical practice, JRP could be considered a warning sign of potential selective IgA deficiency, and therefore of the disorders and abnormalities associated with this disease, such as selective IgG subclass deficiencies, common variable immunodeficiency, recurrent sinopulmonary infections, gastrointestinal disorders (especially coeliac disease), neurologic disorders, autoimmune diseases and even malignant tumours. The IgG subclass levels in our 2 patients were normal or elevated, and no associated diseases were identified in the initial workup or during the follow-up.
Coeliac diseaseThe study by Concheiro et al.,1 from which we obtained the laboratory and imaging test protocol for evaluation of JRP used in our study, included testing for markers of coeliac disease as part of the workup, which would be founded on the potential association of JRP with salivary gland disease resulting from malnutrition secondary to gluten enteropathy, cystic fibrosis or diabetes mellitus. However, none of the 3 patients with coeliac disease in our series, confirmed by the detection of villous atrophy (Marsh score III), 1 of whom received a diagnosis of diabetes mellitus 10 months after, nor another patient with normal villous architecture but with persistently positive tissue transglutaminase and endomysial antibody test results (possible coeliac disease) had any degree of malnutrition. In the 2 years of follow-up, these patients continued to experience episodes of parotitis while on a gluten-free diet. There is no previous description in the literature of a monosymptomatic form of coeliac disease presenting as JRP. We only found one article published in 198314 that described the case of a female patient aged 13 years with JRP and villous atrophy presenting in association with selective IgA deficiency, elevation of ANA, atopic dermatitis, hay fever and blepharitis that received a final diagnosis of gluten enteropathy and who achieved histological recovery and a reduction in the frequency of parotitis episodes with a gluten-free diet. The only reference to her nutritional status was growth delay at age 11 years, while the episodes of parotitis started 1 year after. The author interpreted the findings as immune changes associated with JRP and not secondary to undernutrition. More recently, Gungor et al.15 described a case of JRP associated with Sjögren syndrome and made a literature review of cases associated with immune disorders, including the case of coeliac disease documented by Friis et al.14
Our findings suggest that JRP could be a new indicator to rule out monosymptomatic coeliac disease. Based on the previous evidence, routine screening for coeliac disease could be useful in these patients due to the potential association of JRP with this disease. Routine testing for markers of coeliac disease in patients with JRP could bring forth evidence that this obscure association was not due to chance in future case series.
Sjögren syndromeSjögren syndrome is a progressive autoimmune disease that affects exocrine glands, mainly salivary and lacrimal glands, and presents with xerostomia and xerophthalmia. Although frequently diagnosed in adults, onset in childhood or adolescence is rare and often incomplete, in which context development of recurrent parotitis is a common warning sign. A multicentre study in 40 paediatric patients with a diagnosis of primary SS found that the most common manifestation at onset was JRP, present in 72.5% of cases, while the rest of initial signs and symptoms were nonspecific.16 The authors of this study reviewed smaller case series of similar characteristics published in the past. As was the case in our patients, all patients presented with elevated markers of autoimmune disease, especially ANAs, anti-Ro/SS and anti-La/SSB antibodies, rheumatoid factor and hypergammaglobulinemia. Patient 5 (Table 3) also tested positive of anti-dsDNA antibodies and lupus anticoagulants, had low C4 levels and leukopenia and had arthritis in more than 2 joints, leading to diagnosis of Sjögren syndrome secondary to or associated with systemic lupus erythematosus (SLE). Patient 8 (Table 3) only had elevation of ANAs (1/1280) and hypergammaglobulinemia, and the parotid scintigraphy found features compatible with early-stage SS. The absence of other signs and symptoms and negative results of other tests for immune markers typical of SS could be explained by the young age at diagnosis (4 years) compared to the mean age at diagnosis of cases reported in the literature (9.4–10.7 years).17–19 The heterogeneity of the applied diagnostic criteria and the lack of validation of these criteria in the paediatric age group pose a challenge to the diagnosis of SS at early ages, which requires additional tests, such as salivary gland scintigraphy or biopsy, and a longer follow-up. Gungor et al.15 described one patient that experienced recurrent episodes of parotitis from age 2 years and received a diagnosis of SS at age 4 years despite not meeting the full American-European consensus criteria, something that also occurs in other case series in the literature. Once again, our findings highlight the importance of performing tests for markers of autoimmune disease in patients with JRP, including those younger than 5 years (contrary to the recommendation of Concheiro et al.1), given the importance of early diagnosis of SS to initiate follow-up and treatment by a rheumatologist.
Other immune abnormalitiesIn 20 patients in our series, testing did not identify any serological abnormalities, and they received a final diagnosis of isolated JRP at variable times after onset. Of the 16 remaining patients, 10 have been described in previous sections, and in the other 6, the evaluation found isolated positive titres of ANA (1/40 to 1/120) with or without hypergammaglobulinemia and low levels of C4, with positive thyroid peroxidase antibodies accompanied by elevation of thyroid-stimulating hormone in one patient that received a final diagnosis of Hashimoto disease. A specific autoimmune disorder could not be diagnosed in these patients except the last one, so the remaining 5 patients received a final diagnosis of isolated JRP.
In one of the largest series published to date, Concheiro et al.1 found increased levels of IgE in 2 cases and decreased levels of IgA in 1 case out of a total of 30 cases that met the criteria for JRP. None of the cases was associated with an autoimmune disorder. In another large series published by Leerdam et al.,3 of a total of 50 paediatric patients identified retrospectively in a period of 21 years, 2 had increased levels of ANA, 1 tested positive for antithyroglobulin antibodies and 1 had levels of IgG and IgM in the lower limit of normal. These findings may be biased due to the low percentage of patients that underwent measurement of serum immunoglobulin levels (35.8%) or tests for detection of autoantibodies (24.5%). Of the other 3 patients, 2 received a diagnosis of congenital hypogammaglobulinemia, and 1 a diagnosis of SS with positive tests for ANA, rheumatoid factor and anti-Ro antibodies. In a retrospective 4-year review of cases that included 33 patients, Papadopoulou-Alataki et al.4 did not find any positive autoantibody test results or evidence of autoimmune disease. They only found one patient with selective IgA deficiency and another with isolated IgG4 deficiency.
We are unable to provide data on IgE levels and IgG subclasses because they were not measured routinely in the diagnostic workup. We also cannot conclude that autoimmune disorders and isolated antibody abnormalities are more frequent in patients with JRP compared to the general population, as the series was small and there was no control group, although overall we did find a greater than expected prevalence of final diagnoses related to autoimmunity.
In a prospective case-control study, Wu et al.20 assessed immune function in 100 paediatric patients per group and found significant differences with higher CD4 cell counts and lower CD8 counts and IgG, IgE, IgA and C3 levels in the case group compared to controls. The authors concluded that immune function was altered in patients with JRP compared to the general population, with a reduced cellular immune response and inadequate antibody production. The authors proposed that immunotherapy aimed at improving cellular immunity, and therefore the immune response at the systemic and parotid gland levels, should be a priority in these patients.
Lastly, López Picó et al.21 were the first to describe a case of JRP in association with natural killer (NK) cell deficiency in a girl aged 9 years, but given the benign course of the disease, they did not recommend routine performance of NK cell counts.
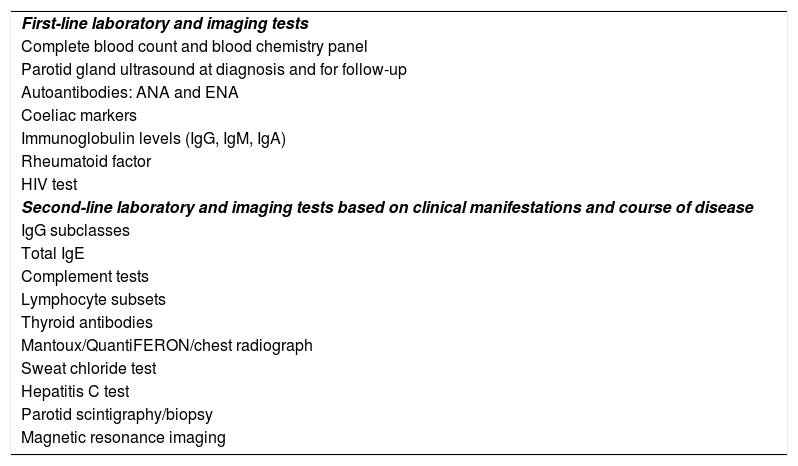
ConclusionsJuvenile recurrent parotitis may be a warning sign of other immune or autoimmune disorders whose early diagnosis and treatment could improve outcomes. A diagnostic workup of JRP should be carried out in every single case (Table 4). Investigation of potential viral aetiologies should not be prioritised, with the exception of HIV testing.
Proposed diagnostic protocol for evaluation of juvenile recurrent parotitis at any age.
| First-line laboratory and imaging tests |
| Complete blood count and blood chemistry panel |
| Parotid gland ultrasound at diagnosis and for follow-up |
| Autoantibodies: ANA and ENA |
| Coeliac markers |
| Immunoglobulin levels (IgG, IgM, IgA) |
| Rheumatoid factor |
| HIV test |
| Second-line laboratory and imaging tests based on clinical manifestations and course of disease |
| IgG subclasses |
| Total IgE |
| Complement tests |
| Lymphocyte subsets |
| Thyroid antibodies |
| Mantoux/QuantiFERON/chest radiograph |
| Sweat chloride test |
| Hepatitis C test |
| Parotid scintigraphy/biopsy |
| Magnetic resonance imaging |
Lastly, patients with JRP and without associated autoimmune disease, with or without nonspecific serological abnormalities, could be eligible for an evaluation of cellular and humoral immunity in the future for therapeutic purposes, although given the benign nature of this presentation and the tendency to resolve spontaneously in puberty, this option would only be considered in patients with frequent episodes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hidalgo-Santos AD, Gastón-Téllez R, Ferrer-Lorente B, Pina-Pérez R, Oltra-Benavent M. Alteraciones inmunológicas asociadas a parotiditis crónica recurrente juvenil. An Pediatr (Barc). 2021;95:260–266.