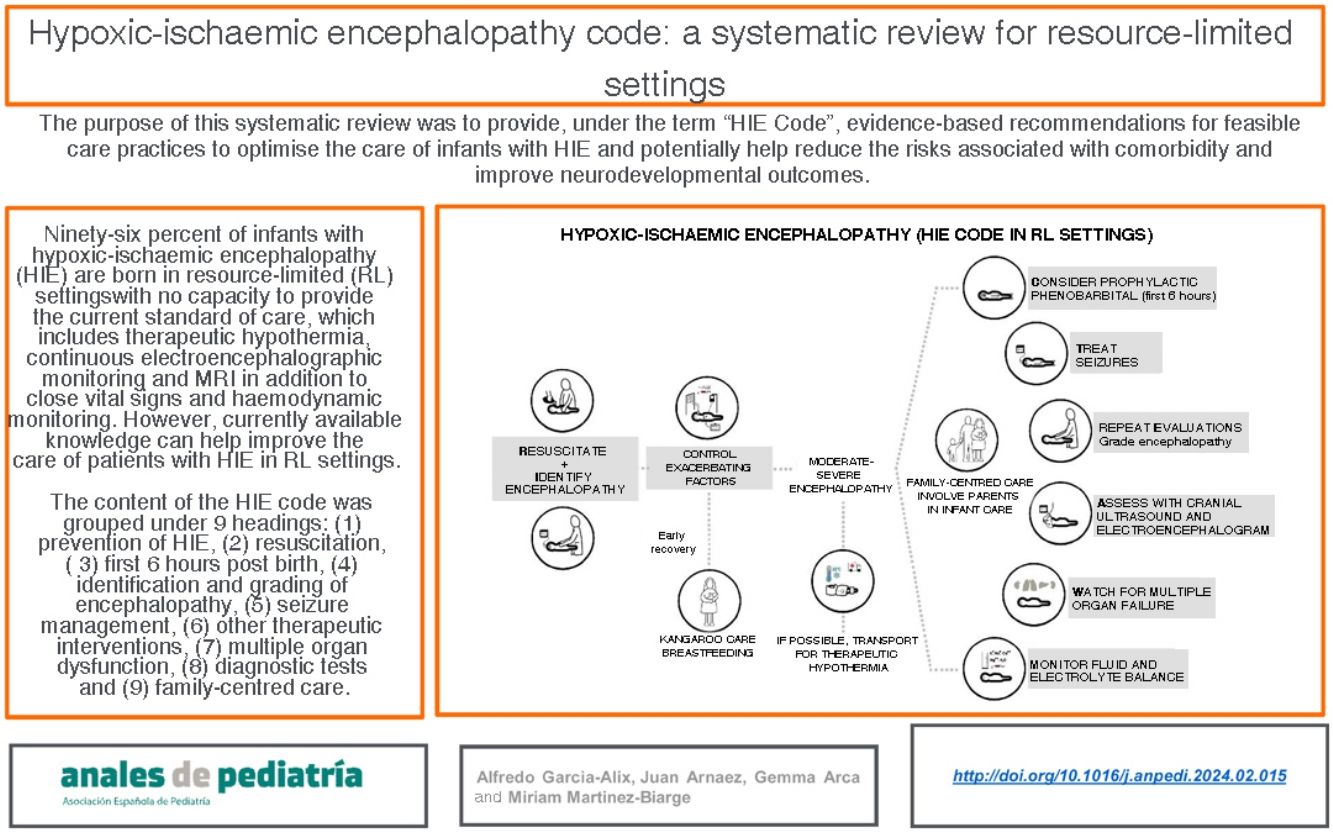
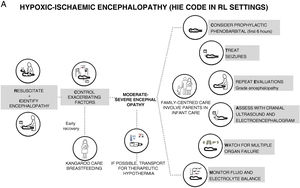
It is estimated that 96% of infants with hypoxic-ischaemic encephalopathy (HIE) are born in resource-limited settings with no capacity to provide the standard of care that has been established for nearly 15 years in high-resource countries, which includes therapeutic hypothermia (TH), continuous electroencephalographic monitoring and magnetic resonance imaging (MRI) in addition to close vital signs and haemodynamic monitoring. This situation does not seem to be changing; however, even with these limitations, currently available knowledge can help improve the care of HIE patients in resource-limited settings. The purpose of this systematic review was to provide, under the term “HIE Code”, evidence-based recommendations for feasible care practices to optimise the care of infants with HIE and potentially help reduce the risks associated with comorbidity and improve neurodevelopmental outcomes. The content of the HIE code was grouped under 9 headings: (1) prevention of HIE, (2) resuscitation, (3) first 6h post birth, (4) identification and grading of encephalopathy, (5) seizure management, (6) other therapeutic interventions, (7) multiple organ dysfunction, (8) diagnostic tests and (9) family care.
Se estima que el 96% de los recién nacidos con encefalopatía hipóxico-isquémica (EHI) nacen en entornos con recursos limitados (ERL) sin capacidad para ofrecer el estándar asistencial vigente desde hace cerca de 15 años en los países con altos recursos y que incluye hipotermia terapéutica (HT), neuromonitorización continua electroencefalográfica y resonancia magnética (RM), además de un control intensivo de las constantes vitales y del equilibrio homeostático. Esta situación no parece estar cambiando; sin embargo y aún con estas limitaciones, el conocimiento actualmente disponible permite mejorar la asistencia de los pacientes con EHI atendidos en ERL. El propósito de esta revisión sistematizada es ofrecer, bajo el término “Código EHI”, recomendaciones de prácticas asistenciales basadas en evidencia científica y factibles en ERL, que permitan optimizar la atención del recién nacido con EHI y ayuden potencialmente a reducir los riesgos asociados a la comorbilidad y a mejorar los resultados neuroevolutivos. El contenido del código EHI se agrupó en 9 epígrafes: 1) prevención de la EHI, 2) reanimación, 3) primeras 6 horas de vida, 4) identificación y graduación de la EHI, 5) manejo de las convulsiones, 6) otras intervenciones terapéuticas, 7) disfunción multiorgánica, 8) estudios complementarios, y 9) atención a la familia.
Hypoxic-ischaemic encephalopathy (HIE) is the main cause of acquired brain injury and disability in term or near-term newborns. It constitutes a considerable health burden in terms of years of life lost and disability-adjusted life years, in addition to having a substantial emotional and economic impact on families and society.1
It is estimated that in year 2010, 1.15 million newborns globally (8.5 per thousand live births) developed HIE, with 96% of cases occurring in low- and middle-income countries (LMICs).2 While in high-income countries (HICs) the incidence of HIE is less than 2 per thousand live births, in LMICs is estimated to be greater than 8 per thousand live births.
The standard of care for the comprehensive management of newborns with HIE in HICs includes therapeutic hypothermia (TH), neuromonitoring with continuous EEG and magnetic resonance imaging (MRI).3,4 However, more than 15 years after the introduction of this standard of care, the reality in LMICs is quite different. Furthermore, while some clinical trials and systematic reviews have found a neuroprotective effect of TH using low-cost equipment in LMICs,5,6 others have not found a decrease in mortality7,8 or have even found deleterious effects with the implementation of this strategy in these countries.9
Several factors may explain the apparent lack of a consistent effect of TH in LMICs, including a lack universal health coverage, deficiencies in prenatal care and the management of complicated deliveries, poor quality in neonatal intensive care due to a scarcity of medical or nursing staff, a high proportion of newborns without access to safe transport to a hospital with adequate resources and gaps in the training and experience of health care professionals, in addition to the ineffectiveness of low-tech cooling devices. Certain population characteristics, such as the rates of infection or of intrauterine growth restriction, may also play a role.2,7
The gap in the care of patients with HIE has been widening between different regions in the world based on income and also between regions within single countries based on the available resources. This is discouraging to many professionals working in resource-limited (RL) settings, who feel that little can be done in the management of HIE, which for them continues to be an “untreatable” disease. However, despite the limitations imposed by the unavailability of TH or neuromonitoring, the potential to improve the care of newborns with HIE in RL settings applying current knowledge may be underestimated.
We prefer to speak of RL settings rather than LMICs, as it is well known that there can be substantial differences in the availability of resources between health care facilities.6 The aim of this document is to offer recommendations for clinical practice, constituting what could be termed a “HIE code”, with the aim of preventing brain damage and improving neurodevelopmental outcomes in infants with HIE managed in LR settings in which TH, neuromonitoring or MRI are not available but there is access to a neonatal intensive care unit (NICU) (Fig. 1). We use the term “HIE code” because HIE is an illness whose impact is time-dependent, so that delays in diagnosis or treatment have a negative impact in its prognosis and outcomes, and in which the implementation of adequate care would require, like the “activation” of a code, an established system for coordinating services at levels of care of varying complexity and the standardization of procedures for consistent implementation by all health care professionals involved in care delivery during the care episode.
Material and methodsWe conducted a systematic search of the literature in Spanish, French or English (PubMed and EMBASE) published through October 31, 2023 using the following medical subject headings (MESH) terms: (“Hypoxic-Ischemic Encephalopathy” OR “Neonatal Encephalopathy” OR “seizures” OR “Brain Magnetic Resonance Imaging” OR “Brain Ultrasound” OR “Electroencephalography”) AND (“Pediatrics” OR “Infant, Newborn” AND “low- and -middle income countries” OR “resource-limited”). Two of the researchers (AGA and JA) identified and selected the studies. If the nature of the identified studies and the absence of randomised controlled trials (RCTs) did not allow a meta-analysis, we conducted a descriptive analysis of the contextual data followed by a discussion among the authors. If, however, there were any RCTs or systematic review (meta-analysis), we developed recommendations according to the level of evidence.
We grouped the evidence on HIE under nine headings: (1) prevention of HIE, (2) resuscitation, (3) first 6h post birth, (4) detection and grading of encephalopathy, (5) seizures, (6) therapeutic interventions, (7) multiple organ dysfunction, (8) diagnostic tests and (9) family-centred care.
Operational definitionsPerinatal asphyxia: abnormal cardiotocographic pattern, complicated birth or sentinel event requiring advanced resuscitation at birth and/or 5-minute Apgar score of 5 or less and/or cord blood pH of 7.00 or less or base deficit greater than 16mmol/L.
Perinatal HIE: neurologic impairment (encephalopathy) from the first hours post birth attributable to a hypoxic-ischaemic attack around the time of birth and associated with a history of perinatal asphyxia.
ResultsPrevention of hypoxic-ischaemic encephalopathyAlthough there are antepartum factors that may predispose or contribute to intrapartum adverse events, their presence alone is not sufficient to cause HIE, and the intrapartum period is considered the time when the risk is highest, with intrapartum events constituting the main or a contributing factor in the causal pathway of HIE.10 The aim of obstetric care is to identify and rescue the foetus at risk of hypoxic-ischaemic events through the recognition of signs suggestive of foetal hypoxia. The high rates of HIE in LMICs are probably determined, in part, by social factors that affect maternal health and by the circumstances and the setting of childbirth. Delivery of higher-quality prenatal care by qualified community-based midwives and the reinforcement of hospital and out-of-hospital maternal and neonatal care systems could achieve reductions in perinatal mortality and the incidence of HIE.11
ResuscitationThe goal of neonatal resuscitation in newborns with perinatal asphyxia is to re-establish adequate tissue oxygenation and cerebral blood flow and to prevent conditions or events affecting the brain and other organs that could exacerbate the hypoxic-ischaemic insult. It is estimated that 15% of newborns in LMICs require basic resuscitation (stimulation, “sniffing” positioning of head, opening of airway and bag-mask ventilation with room air), which reduces intrapartum mortality by 40%, neonatal intrapartum-related mortality by 30% and early neonatal mortality by 38%.12
Competence in neonatal resuscitation acquired through training programmes is crucial to guarantee the safety and effectiveness of this intervention and to reduce morbidity and mortality secondary to perinatal asphyxia, which are particularly high in RL settings.12
The International Liaison Committee on Resuscitation (ILCOR) periodically publishes consensus-based recommendations that are the foundation of the three most widely used neonatal resuscitation guidelines and training programmes worldwide: the neonatal resuscitation programme, neonatal life support and Helping Babies Breathe (HBB), especially developed for LR settings. Table 1 summarises the key points of current recommendations, in reference to the 2020 ILCOR consensus,13 the 2021 European Resuscitation Council Guidelines,14 the 2023 American Heart Association and American Academy of Pediatrics guidelines15 and, in Spanish, the specific position statement of the Neonatal Resuscitation Group of the Sociedad Española de Neonatología (SENeo, Spanish Society of Neonatology)16 as well as, on the subject of basic resuscitation in RL settings, the second edition of the HBB from 2016.
Recommendations for neonatal cardiopulmonary resuscitation (these recommendations are not to replace local neonatal resuscitation guidelines that take into account the resources available at any given time).13–16
| 1. At least one health professional (doctor, nurse or midwife) with neonatal resuscitation skills should be present in each delivery. |
| 2. The room temperature in the setting of neonatal resuscitation should not exceed 23°C to prevent maternal and neonatal hyperthermia. |
| 3. The HBB programme was developed specifically for RL settings in which transcutaneous SatO2 monitors, advanced thermoregulation equipment, and humidified and heated gases are not available. This programme allows training of professionals with different qualifications, including doctors and hospital and community midwives. |
| 4. Initiate CPR with heated and humidified room air (FiO2 0.21) and consider administration of oxygen at increasing concentrations based on pulse oximeter reading (right hand), the interpretation of the clinical response (assessment of colour of skin and mucosae/cyanosis) is subject to significant bias and should only be used when pulse oximetry is not available. |
| 5. Avoid hypoxia and hyperoxia. Current recommendations apply the Dawson charts (2010) for reference; the SatO2 of healthy newborns increases progressively after birth: 60%–65% at 1min, 65%–70% at 2min, 70%–75% at 3min, 75%–80% at 4min, 80%–85% at 5min and 85%–95% at 10min maintaining a HR>100bpm. Studies that implemented delayed cord clamping found a quicker increase in O2 saturation. |
| 6. For CPR, the compression to ventilation ratio should be 3:1 using 100% O2 and reducing the concentration gradually once the HR recovers. The HR should be monitored with ECG or, if ECG is not available, with auscultation and pulse oximetry. |
| 7. In the case of an unresponsive baby born with meconium-stained fluid, it is better to initiate CPR without examining the airway with a laryngoscope and without tracheal suctioning. Intubation and tracheal suctioning are very rarely needed to manage potential airway obstruction. |
| 8. If a HR greater than 60 bpm is not achieved with ventilation and compressions, administer adrenaline at a dose of 0.01−0.03mg/kg intravenously (umbilical vein); repeating the dose every 3−5min if needed. Administer doses of 0.05−1.00mg/kg tracheally until vascular access is established. |
| 9. Avoid volume expansion (crystalloids or packed red blood cells) except in evident cases of hypovolaemia that do not respond to CPR. |
| 10. Avoid the use of bicarbonate save in cases of prolonged CPR without response. |
| 11. While delayed cord clamping (≥30s) is recommended in term or near-term infants, if the newborn requires resuscitation, initiation of CPR manoeuvres should not be delayed to defer cord clamping.a |
| 12. If the patient can be transported to a facility with TH, there is the option of turning off the exothermic mattress in the resuscitation platform once the HR has normalised, monitoring the central (rectal) temp closely or, if not possible, measuring the axillary temp every 15min and preventing temperatures of less than 33°C or greater than 36.5°C. |
| 13. Consider discontinuation of resuscitation manoeuvres from 10min after their initiation and especially after 20min. |
CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; FiO2, fraction of inspired oxygen; HBB, Helping Babies Breathe; HR, heart rate; min, minute; RL, resource-limited; s, seconds; SatO2, oxygen saturation; T, temperature; TH, therapeutic hypothermia.
A trial that compared delayed cord clamping with umbilical cord milking in nonvigorous newborns delivered at 35 and 42 weeks of gestational age (limp, pale, and minimal or no breathing in the first 15s after birth) found that newborns in the cord milking arm had higher haemoglobin levels, a lower probability of an abnormal 1-minute Apgar score, received less delivery room cardiorespiratory support, had a lower incidence of moderate or severe HIE and received less TH. These outcomes need to be assessed in additional studies before introducing this intervention.
The control of comorbid factors that can exacerbate hypoxic-ischaemic brain lesions is a cornerstone in the care of newborns with HIE and is particularly important in RL settings. The recommendations described in this section apply to the immediate postpartum period in a newborn with perinatal asphyxia, but can also be extended to the first few days of life in newborns with HIE. Monitoring and control of body temperature and blood glucose levels (Appendix A, Table S1) are key, in addition to other aspects presented in Table 2.
Recommendations for the management of comorbid factors in the first 6h post birth in newborns with asphyxia/HIE.
| (A) Temperature |
| 1. Treat maternal hyperthermia with antipyretic drugs or physical measures and consider the presence of obstetric infection, especially in settings without epidural anaesthesia. |
| 2. In the first 6h post birth in a newborn with neonatal asphyxia, record the temp every 30min while assessing for the presence of HIE. |
| 3. Ideally, continuous central temp monitoring or, otherwise, intermittent central temp measurements (inserting probe in rectum at a depth of 5cm) of, if not possible, monitoring of peripheral temp during the first 72h post birth and especially in the first 24h. |
| 4. In the case of hyperthermia (central temp > 37°C) at admission, turn off external heat sources and, if needed, use physical measures to reduce temp. |
| 5. In the case of spontaneous hypothermia (central temp < 36°C) at admission, avoid overcooling (central temp < 33°C) and allow infant to warm up gradually and slowly (<0.5°C an hour) to normothermia, without actively warming the infant. |
| (B) Blood glucose |
| 1. In asphyxiated newborns with neural and cardiorespiratory function recovery and resolution of lactate acidosis, blood glucose levels need to be monitored in the first 6h post birth to prevent hypoglycaemia (<46mg/dL) along with initiation of breastfeeding. |
| 2. In infants with HIE, blood glucose levels must be monitored throughout the first 72h post birth, with particular emphasis on the first 24h: |
| • Prevent hypoglycaemia. Initiate IV infusion of glucose at 6−8mg/kg/min and maintain it for the first few hours of life. |
| • If hypoglycaemia persists despite IV infusion, increase dose to 2mg/kg/min. Check blood sugar level every 30−60min until the level reaches 50mg/dL, after which the levels should be checked every 4−6h. |
| • In the case of hyperglycaemia (>150mg/dL), reduce the amount of glucose administered in IV solution. Avoid administration of insulin unless there is persistent glucose elevation that does not respond to reducing the dose. |
| (C) Other factors |
| 1. Avoid severe hypocarbia (pCO2<20mmHg) and sustained moderate hypocarbia (pCO2 20−25mmHg). |
| 2. Avoid hypoxia and hyperoxia (SatO2<92% or >98%). |
| 3. Avoid hypotension (MAP<40mmHg) and abrupt changes in BP. There is a close association between systemic and cerebral haemodynamics and loss of autoregulation of CBF in newborns with HIE. |
| 4. Avoid volume expansion and bicarbonate. |
| 5. Correct electrolyte abnormalities. |
BP, blood pressure; CBF, cerebral blood flow; HIE, hypoxic-ischaemic encephalopathy; MAP, mean arterial pressure; pCO2, partial pressure of carbon dioxide; SatO2,oxygen saturation; temp, temperature.
The identification HIE and grading its severity are key in the management of newborns with perinatal asphyxia (Table 3 and Appendix A S2).
Identification and grading of severity of encephalopathy.
| (A) Usefulness of detecting and grading the severity of encephalopathy |
| 1. Identifying patients eligible for transport to a facility with TH. |
| 2. Anticipating the potential severity of multiple organ dysfunction that may develop in the next few hours and days. |
| 3. Establishing the required level and duration of monitoring and surveillance. |
| 4. Establishing an adequate prognosis. |
| 5. Predicting the probability of damage in neuroimaging tests. |
| 6. Establishing neurodevelopmental risk groups and optimising the use of resources during follow-up. |
| (B) Recommendations |
| 1. Perform neurologic exams and grade the severity of HIE in the first 6h, at 48h, between 72−96h, at 7 days and before discharge using one of the available specific scales. |
| 2. There are scales that reflect the full spectrum of severity of HIE more accurately, like the Thompson, NICHD, SIBEN or García-Alix scores. The latter provides descriptions of standardised manoeuvres and potential responses to facilitate the application and interpretation of the scale (Appendix A Table S2) |
| 3. Training on how to assess the severity of encephalopathy with available resources: |
| 3.1 Laminate the document of the scale, which should include clear operational definitions, and keep it close to the infant to make it easily accessible. |
| 3.2 Film the patient (after obtaining consent from the parents) and discuss observations among the staff and with a trained colleague. |
| 3.3 Develop educational material on the neurologic exam that must be performed along with clinical examples of the presence and severity of HIE. Provide periodic training and update/refresher trainings. |
| 3.4 Have a specialist with specific training in neonatal neurology on staff. |
HIE, hypoxic-ischaemic encephalopathy; NICHD, National Institute of Child Health and Human Development; TH, therapeutic hypothermia; SIBEN, Sociedad Iberoamericana de Neonatología.
The severity of HIE in the first 6h post birth is the key factor in the selection of candidates for TH. In the era before TH, the level of impairment associated with HIE was low if the encephalopathy was mild, intermediate if the encephalopathy was moderate and high if the encephalopathy was severe (practically all infants with severe HIE died in the neonatal period or had severe disability at 18–24 months).17–19 Similar outcomes were reported in RCTs that assessed the efficacy of TH.20–22 While the predictive value of the severity of HIE in the first 6h is moderate for the prediction of long-term outcomes (Appendix A Table S3), the severity assessed at 48–96h and at 7 days offers a more accurate prognosis,19,23–25 which reflects the dynamic nature (improvement or worsening) of HIE.18,19,23,24 When the encephalopathy does not improve in the first 72h, the probability of death or disability is much higher than when it improves within 24h post birth. In RL settings, the assessment of severity of HIE as well as changes in severity over time, using categorical or numerical scales,19,26 can be the most efficient approach for establishing neurodevelopmental risk groups. If the staff is insufficiently trained, it is best to seek consultation with local experts or through telemedicine.27
SeizuresHypoxic-ischaemic encephalopathy is the leading cause of neonatal seizures independently of the income and resources of the country.28 In HICs, 50%–85% of newborns with moderate or severe HIE develop clinical seizures, whether or not they are managed with cooling,22,23,29 compared to nearly 80% of patients with moderate HIE and more than 80% of patients with severe HIE in RL settings.5,9
The onset of seizures associated with HIE usually occurs after the first 6h post birth, with onset occurring between 6 and 24h post birth in half the patients and commonly after 12h.29–31 This suggests that when features suggestive of seizures are observed before 6h, they may not be actual seizures or may be seizures secondary to a prenatal hypoxic-ischaemic insult or with a different aetiology.
When neonatal seizures in infants with HIE are only identified based on clinical features (without the use of amplitude-integrated electroencephalography [aEEG]), the true seizure burden (seizure frequency and cumulative duration over a given period) is underestimated, as most neonatal seizures are subclinical and up to 23% of these patients exhibit electrographic status epilepticus in the first days of life.29,31 On the other hand, even experienced clinicians have difficulty distinguishing convulsive seizures from non-epileptic motor phenomena based on observation alone, so there is a risk of overdiagnosis of neonatal seizures. In consequence, the International League Against Epilepsy (ILAE) has underscored the importance of EEG monitoring for the accurate diagnosis of neonatal seizures.32
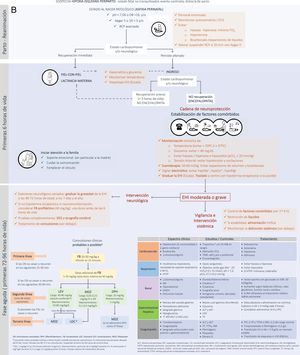
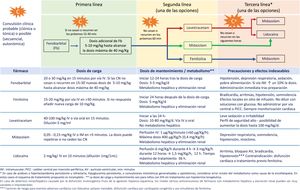
The seizure burden in newborns with moderate to severe HIE managed with or without TH is high, is dependent on the severity of HIE and peaks around 24h post birth.29,30 The seizure burden is also an important predictor of lesions in neuroimaging and of neurodevelopmental disorders, and its association with adverse outcomes is independent of the severity of HIE and the use (or lack thereof) of TH.33,34 Therefore, prompt and effective treatment of neonatal seizures is of the essence (Fig. 2, Table 4, Appendix A S4). Treatment algorithms must call for caution in the approach to refractory seizures, broadening the differential diagnosis to include aetiologies different from or concurrent with hypoxia-ischaemia (Fig. 2).
Recommendations for the management and treatment of neonatal seizures in infants with HIE in resource-limited settingsa.
| 1. Initiate antiepileptic treatment with phenobarbital without delay in infants with clinical seizures with focal clonic or tonic features. |
| 2. Consider antiepileptic treatment with phenobarbital in infants with clinical sequential, autonomic or generalised myoclonic seizures. |
| 3. If monitoring is not available, actively treat with antiepileptic drugs until the full resolution of clinical seizures. Since it is not possible to ascertain the resolution of electrographic seizures, a high index of suspicion must be maintained. |
| 4. Do not initiate treatment in patients with automatism or episodes of generalised tonic extension sensitive to stimulation (“posturing”). |
| 5. It is recommended that each facility develops a management protocol contingent on the available resources, choosing among the options shown in Fig. 2. |
| 6. Initiate the gradual withdrawal of antiepileptic drugs once the patient has been free of seizures for 72 consecutive hours. The goal, if possible, is to discontinue treatment completely before discharge. |
| 7. In infants who have required more than one antiepileptic drug, we recommend reducing the number of antiepileptic drugs starting with third-line drugs, with subsequent discontinuation of second-line drugs if the patient remains seizure-free for 48–72h, and removing phenobarbital (the first-line drug) last. |
| 8. Assessment of the patient with conventional EEG is recommended after the discontinuation of each antiepileptic drug. |
EEG, conventional electroencephalogram; h, hours; HIE, hypoxic-ischaemic encephalopathy.
We recommend consulting Tables S4 and S5 in Appendix A for more detailed information on neonatal seizures.
Since many neonatal units in RL settings have limited access to continuous EEG monitoring or aEEG, treatment of neonatal seizures depends on their identification based on clinical features, on which health care professionals must therefore be trained. The Brighton Collaboration has proposed a has proposed a scheme with five levels of diagnostic certainty that can guide treatment decisions and help avoid overtreatment if EEG is not available35 (Appendix A Table S5).
On the other hand, continued exposure to antiepileptic drugs can have noxious effects on the developing brain in the long term and, due to the sedative effects, delay the establishment of oral feeding and hinder bonding between the newborn and the parents. Therefore, antiepileptic treatment should only be given for as long as they are needed.
There is limited consensus regarding the duration of antiepileptic treatment once the seizures have ceased.32,36 However, there is growing evidence that neonatal seizures in infants with HIE are rare past day 4 or 5 post birth and that recurrence is also infrequent once they are controlled.29,31
Therapeutic interventionsDelivery of TH for 72h using passive cooling or manual (not servo-controlled) active cooling is not recommended. These interventions should be restricted to very short periods with close monitoring of body temperature, for instance, for induction of hypothermia prior to transport to a facility where servo-controlled cooling is available.7,8
With the exception of TH, the evince on neuroprotective interventions is scarce, and most of the published studies address their feasibility rather than their efficacy. Since the introduction of TH, most of these interventions have been assessed as adjuvants of TH, which limits our knowledge of their performance as monotherapy in RL settings. The only intervention that has proven beneficial in RL settings is the administration of phenobarbital for prophylaxis (Appendix A Table S6).
Multiple organ dysfunctionMultiple organ dysfunction is frequent and its extension and severity are associated with those of HIE and with neonatal mortality.37 It can also cause significant morbidity and exacerbate the brain injury resulting from the hypoxic-ischaemic insult. The monitoring and treatment methods proposed in Table 5 are not based on evidence, as few studies have compared diagnostic or therapeutic interventions. Notwithstanding, systemic support cannot be neglected. The evidence on the close association between systemic and cerebral haemodynamics has motivated the widespread implementation of noninvasive regional cerebral oxygen saturation monitoring and functional echocardiography. The availability of an ultrasound machine can guide the identification and finetuning of the most appropriate treatment.
Summary of clinical features, testing, monitoring and treatment of multiple organ dysfunction.
| Clinical features | Testing/monitoring | Treatment | |
|---|---|---|---|
| Cardiovascular | • Hypotension (↓contractility or ↓cardiac output | • Troponin T and/or CK-MB in blood | • Dobutaminea |
| • Bradycardia | • Abnormal ECG | • Adrenaline | |
| • ↓urine output/oliguria | • MAP, pre- and postductal SatO2 | • Dopamine | |
| • Echocardiography | • Milrinoneb | ||
| Respiratory | • Respiratory failure, need of ventilation or O2 | • Blood gas analysis | • Prevent hypoxia or hyperoxia |
| • PPHN | Objective: anion gap [Na+]−([Cl−]+[CO3H−]) < 15 mEq/L; pH>7.3; pCO2 37.5–52.5mmHg | • Prevent hypocarbia | |
| • If PPHN: milrinone, sildenafilc | |||
| Renale | • ↓urine output/oliguria | • Urine output/fluid balance | • Start supplementation with glucose 10% w/v |
| • ARF | • Cystatin Cd | • Increase based on fluid balance, blood glucose, renal and cardiac function | |
| • Hyponatraemia | • Creatinine | • If ARF refractory to medical treatment: peritoneal dialysis | |
| • SIADH | • Renal ultrasound | ||
| • Renal tubular dysfunction | • Urine electrolytes, specific gravity, osmolality | ||
| Gastrointestinal Hepaticf | • Delayed gastric emptying | • Gastric residual volume and pH (Merck strip) | • Nil per os or trophic feedings (colostrum or breast milk 1−3mL/kg every 3h) |
| • Dysmotility | • Blood glucose | • Consider administration of vitamin K or FFP | |
| • Gastric bleeding | • ALT and AST | ||
| • Hypoglycaemia | • LDH | ||
| • ↑AST/ALT (> 100 IU/L) | • PT | ||
| Coagulopathy | • Thrombocytopenia | • ALT and AST | • FFP if PT or aPTT or INR>1.5 ULN |
| • Coagulopathy | • PT, aPTT, INR | • Cryoprecipitate if fibrinogen > 1.5g/L | |
| • Active bleeding (especially pulmonary and gastrointestinal) | • Fibrinogen | • pRBC if Hb < 7−8g/dL in stable infant | |
| • Platelets | • Platelet transfusion if count < 50 000/mL | ||
| • D-dimer |
ALT, alanine aminotransferase; aPPT, activated partial thromboplastin time; ARF, acute renal failure; AST, aspartate aminotransferase; pRBC, packed red blood cells; ECG, electrocardiogram; FFP, fresh frozen plasma; Hb, haemoglobin; INR, international normalised ratio; LDH, lactate dehydrogenase; MAP, mean arterial pressure; pCO2, partial carbon dioxide pressure; PPHN; persistent pulmonary hypertension of the newborn; PT, prothrombin time; SatO2, oxygen saturation; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Dobutamine may be the drug of choice due to its α agonist effects at low doses without increasing vascular resistance. In addition, adrenaline is a good option due to its action on α and β2 receptors and the reduction of pulmonary vascular resistance and is particularly indicated in PPHN, when it is necessary to increase systemic vascular resistance.
It is easier to detect decreases in the glomerular filtration rate by measuring the serum level of cystatin C than measuring the concentration of creatinine.
Exercise caution when using nephrotoxic drugs, such as aminoglycosides, nonsteroidal anti-inflammatories and vancomycin. Cefotaxime can replace gentamicin since it covers a similar spectrum, but without the nephrotoxic effects. In patients with acute renal failure, consider reducing the dose of antiepileptic drugs excreted by the kidney (phenobarbital, hydantoin, midazolam, lidocaine).
Fluid administration must be carefully controlled. Since infants with HIE can have delayed micturition, acute renal failure and, in some cases, syndrome of inappropriate antidiuretic hormone secretion (SIADH), they are at risk of fluid overload and hyponatraemia. Fifteen to forty percent of non-cooled infants with HIE have abnormal renal function test results and more than 30% have hyponatraemia.
More than 50% of newborns with HIE exhibit additional electrolyte abnormalities.20,22,38 The most frequent ones are, in decreasing order, hypocalcaemia, hyperkalaemia, hypokalaemia, hyponatraemia e hypomagnesemia.22 We recommend monitoring of serum electrolytes every 12–14h (Table 6 and Appendix A S7).
Recommendations for fluid and electrolyte management and nutrition in newborns with HIE.
| 1. In NBs with HIE, administration of fluids and electrolytes can be harmful and worsen brain injury. |
| 2. Fluid overload and hyponatraemia can contribute to the development of cerebral oedema. Light fluid restriction is recommended (55−60mL/kg/day) until a urine output of 1mL/kg/hour is achieved.3 |
| 3. Fluid restriction in the first 72h must allow maintenance of a neutral or slightly positive fluid balance, administration of an adequate amount of glucose (4−8mg/kg/minute), a serum Na+ level of 135–145mEq/L and a urine output > 1mL/kg/h. |
| 4. In the case of hyponatraemia, establishing the cause is crucial to determine the most appropriate management. Consider the following in the differential diagnosis: excessive administration of fluids (dilutional hyponatraemia), acute renal failure, inadequate secretion of antidiuretic hormone and/or salt wasting syndrome. |
| 5. If fluid restriction is excessive, it can cause dehydration and hypotension, reducing cerebral perfusion and increasing brain damage or reducing the urine output and worsening renal function. |
| 6. If imbalance is detected for any electrolytes, treat immediately followed by maintenance treatment to maintain levels in the normal range (it is preferable for magnesium levels to be in the high-normal range). |
| 7. In NBs with moderate HIE, initiate trophic feedings (10−20mL/kg/day) with human milk and increase volume progressively watching for signs of intolerance, such as abundant, bilious or bloody gastric residuals. |
HIE, hypoxic-ischaemic encephalopathy; Na+, sodium sodio; NB, newborn.
As regards nutrition in newborns with HIE, the RCTs that assessed the efficacy of TH had patients under nil per os for the first 72h due to the risk of intestinal ischaemic-reperfusion injury.38 However, the incidence of severe intestinal damage is very low, and trophic and progressive feedings do not seem to be associated with an increase in intestinal complications (Table 6 and Appendix AS7).
The routine use of antibiotherapy in patients with neonatal asphyxia or HIE is not justified, although a high index of suspicion must be maintained along with close monitoring of markers of infection in blood and cerebrospinal fluid (CSF), especially in patients with a history of chorioamnionitis, fever, elevation of acute phase reactants or in whom the course of neurologic impairment differs from what has been expected.
Diagnostic testsTwo widely available methods, the conventional EEG and the cranial ultrasound (CUS) can be particularly helpful in establishing the prognosis in RL settings (Appendix A Table S8).
Conventional electroencephalogramBefore the introduction of continuous EEG monitoring, severe background pattern disturbances in the EEG in the first days of life had been found to be strongly correlated to the severity of HIE, MRI abnormalities and a poor neurodevelopmental outcome.39–41 This traditional neurophysiological assessment method requires a minimum of 15min; it can be implemented at the bedside, using a limited number of electrodes, and it is relatively easy to interpret.42
Cranial ultrasoundCranial ultrasound is a noninvasive imaging test that is performed at the bedside and can be repeated to observe the evolution of detected abnormalities. Modern equipment with grayscale imaging and probes with variable frequencies have improved the sensitivity and specificity of the diagnosis of lesions by means of CUS. In addition, the widespread availability and affordability of portable ultrasound machines has made CUS increasingly feasible in RL settings. The performance and interpretation of CUS by trained neonatologists has improved access and reduced the cost of this technique.43
In infants with HIE, CUS abnormalities between 24 and 72h have been found to correlate to post-mortem findings44 as well as MRI findings in the first week, and are predictive of a poor outcome.45 Repeated scans allow monitoring of the changes in the identified lesions and defining the pattern and severity of brain injury. In infants with HIE that do not receive TH, the resistive index measured in the anterior cerebral artery is a good prognostic factor.
Family-centred care during the acute phase of encephalopathyThis cornerstone of the comprehensive care of infants with HIE has received little attention until TH was introduced in clinical practice. The birth and hospitalization of infants with HIE is a stressful and traumatic experience for the family. Factors like the technology surrounding the infant, the fear that the infant may die and the uncertainty about the prognosis give rise to feelings of guilt, sadness and hopelessness in parents. In addition, the separation from the infant, the inability or difficulty to hold the infant or to be involved in the infant’s care are factors that hinder parent-child bonding and the development of parental roles, and have a negative impact on the emotional wellbeing of the family (Appendix A Table S9).
ConclusionEven when TH is not available in RL settings, there is evidence in support of other therapeutic approaches that can be accessed in these settings, have a neuroprotective effect and could improve outcomes in these patients. In fact, post-TH cohort studies have found significantly better outcomes compared to RCTs, which suggests that the optimization of intensive care may actually contribute significantly to the improvement that has been attributed to TH.46
This document is the first to propose a code and protocol for the management of HIE, an illness whose outcomes are time-dependent, in RL settings in which TH is not available but neonatal intensive care is. Although there are still issues to be resolved and further research needs to be conducted in RL settings, the implementation of these recommendations could reduce the morbidity and mortality associated with HIE and help use available resources more efficiently.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.