The goal of treatment of type 1 diabetes (T1D) is to achieve optimal glycaemic control to prevent acute and chronic complications of the disease without compromising the quality of life of children and their families.1 However, achieving adequate metabolic control is challenging and requires significant engagement of the family. Despite technological advances, such as continuous glucose monitoring (CGM) and continuous subcutaneous insulin infusion (CSII) systems, T1D has a significant impact on daily life for patients and their immediate circles, increasing the risk of mental health problems.2
Hybrid closed loop systems (HCLSs) consist of an insulin infusion system (or pump), a CGM device and a control algorithm that allows the delivery of variable doses of insulin determined by the algorithm based on the interstitial glucose levels, which reduces to some degree the burden that the disease places on the caregivers (Appendix A).
The objective of our study was to analyse whether there was an improvement in health-related quality of life (HRQoL) of children and parents after initiation of the HCLS.
We conducted a retrospective and prospective study in children with T1D who started treatment with HCLS in a secondary care hospital. The assessment of HRQoL consisted in the administration of the first questionnaire specifically developed for children and adolescents with T1D validated in Spanish (DISABKIDS).3 We retrieved data from the questionnaire completed by the parents and also the questionnaire completed by patients if they were aged more than 8 years, administered at baseline before initiation of the HCLS and again after a minimum of 6 months. We also collected clinical data through the retrospective review of the patients’ health records and the software of the CGM and CSII systems (LibreView, CareLink and Glooko). The main outcomes under study were the questionnaire scores and the measured CGM-derived metrics and glycated haemoglobin (HbA1C). The study was approved by the competent research ethics committee and we obtained informed consent for all participants.
The DISABKIDS questionnaire assesses mental (independence and emotion), social (inclusion and exclusion) and physical (limitation and treatment) dimensions of HRQoL and was developed for assessment of children and adolescents with chronic diseases. We used the diabetes-specific module, which consists of 2 scales: impact and treatment. The former assesses the emotional impact of daily glycaemic control and dietary restrictions. The treatment scale deals with the constant need to plan treatment and carrying equipment. The score reflects a better quality of life the closer it is to 100 (range, 0–100). The questionnaires also include 3 items that assess decompensations in the past year.
The study included 25 patients (56% male). The mean age at diagnosis of T1D was 6.5 years (SD 3.5). Most patients used the Minimed 780G-Guardian 4 system (Medtronic) and 4 the Tandem t:slim X2-Dexcom G6 system (Novalab).
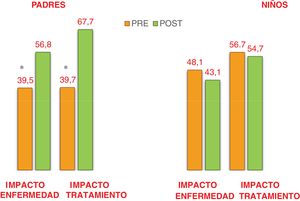
Table 1 presents the CGM-derived metrics and HbA1C values before and after initiation of the HCLS: there was an increase in the number of patients who met the criteria for adequate glycaemic control established by the International Consensus of Time in Range (2019),4 in addition to a significant increase of the time in range, with a decrease in the time with levels between 181 and 250mg/dL and a reduction in the mean glucose level. Fig. 1 presents the scores of parents and children in the pre- and post-HCLS DISABKIDS questionnaires.
Summary of clinical and glucose level data before and after initiation of hybrid closed-loop system.
| Variable (n=25) | Pre-HCLS (mean±SD) | Post-HCLS (mean±SD) | P | Targets established in consensus4 |
|---|---|---|---|---|
| Age (years) | 10±3.5 | 11.5±3.4 | ||
| Insulin dose (U/kg/day) | 0.7±0.3 | 0.8±0.2 | .07 | |
| Mean glucose (mg/dL) | 153.8±16.8 | 145.6±13.5 | .04 | <154b |
| SD | 52.0±11.8 | 48.6±9.2 | .13 | – |
| CV (%) | 33.3±5.5 | 32.9±4.6 | .78 | <36 |
| GMI (%) | 7.0±0.4 | 6.7±0.4 | .05 | <7b |
| Time < 54mg/dL (%) | 0.2±0.5 | 0.4±0.8 | .52 | <1% |
| Time 54−69mg/dL | 1.9±1.8 | 2.2±1.4 | .45 | <4% |
| Time 70−180mg/dL (%) | 69.7±12.7 | 75.7±8.6 | .04 | >70% |
| Time 181−250mg/dL (%) | 23.9±8.9 | 18.0±6.3 | .01 | <25% |
| Time >250mg/dL (%) | 6.0±9.4 | 4.0±3.2 | .29 | <5% |
| HbA1C (%) | 6.8±0.7 | 6.8±0.5 | .82 | <7b |
| Met adequate glycaemic control criteriaa | 28% | 60% | .045 |
CV, coefficient of variation; GMI, glucose management indicator; HCLS, hybrid closed loop glucose monitoring and insulin delivery system; HbA1C, glycated haemoglobin; SD, standard deviation.
We observed improvement in patient CGM-derived metrics and parental HRQoL, which was consistent with previous studies.5,6 However, we did not find significant differences in the HRQoL scores in the children, which were also lower compared to previous reports.3 This could be due to the lower age of patients in our study, as in many cases responsibility for the management of diabetes had yet to be transferred to the patient (self-care), so the burden of diabetes management still fell primarily to the family.
We did not find statistically significant differences between the scores of patients who met the criteria of adequate control and those who did not. This finding is concerning, as patients with poor glycaemic control may perceive their health status as “good” despite a lack of objective indicators to corroborate it (glucose or HbA1C levels), which in turn may affect their adherence to treatment.
The main limitations of our study were the small sample size and the limited duration of treatment with a HCLS. Notwithstanding, it evinced an improvement in CGM-derived metrics as well as in HRQoL in the family. We believe that the HRQoL of patients with T1D and their families should be assessed routinely, and the diabetes-specific module of the DISABKIDS questionnaire can be a suitable tool for the purpose.
Conflicts of interestBelén Huidobro Fernández has received fees for participating in conferences organised by Air Liquid and for attending courses organised by Medtronic. All other authors have no conflicts of interest to disclose.
We thank the participating children and families for their willingness to contribute to this study.