The evaluation of clinical and analytical parameters as predictors of the final growth response in Turner syndrome patients treated with growth hormone.
Material and methodsA retrospective study was performed on 25 girls with Turner syndrome (17 treated with growth hormone), followed-up until adult height. Auxological, analytical, genetic and pharmacological parameters were collected. A descriptive and analytical study was conducted to evaluate short (12 months) and long term response to treatment with growth hormone.
ResultsA favourable treatment response was shown during the first year of treatment in terms of height velocity gain in 66.6% of cases (height-gain velocity >3cm/year). A favourable long-term treatment response was also observed in terms of adult height, which increased by 42.82±21.23cm (1.25±0.76 SDS), with an adult height gain of 9.59±5.39cm (1.68±1.51 SDS).
ConclusionsPredictors of good response to growth hormone treatment are: (A) initial growth hormone dose, (B) time on growth hormone treatment until starting oestrogen therapy, (C) increased IGF1 and IGFBP-3 levels in the first year of treatment, and (D) height gain velocity in the first year of treatment.
Evaluación de parámetros clínicos y analíticos que actúen como predictivos de respuesta al tratamiento con hormona de crecimiento (rhGH) a largo plazo en pacientes con síndrome de Turner.
Material y métodosEstudio retrospectivo de 25 niñas diagnosticadas de síndrome de Turner, de las cuales 17 recibieron tratamiento con rhGH y fueron controladas hasta alcanzar la talla adulta. Se determinaron diferentes variables auxológicas, analíticas, genéticas y farmacológicas a lo largo de su seguimiento en dichas consultas. Se realizó un estudio descriptivo y analítico mediante regresión lineal, con valoración de la respuesta al tratamiento a corto (12 meses) y a largo plazo.
ResultadosSe observó una respuesta favorable a corto plazo valorada en ganancia de velocidad de crecimiento en el 66,6% de los casos (velocidad de crecimiento>3cm/año a los 12 meses de tratamiento respecto a la previa). También se evidenció una respuesta favorable a largo plazo, valorada en una ganancia de talla total de 42,82±21,23cm (1,25±0,76 SDS). Las pacientes ganaron una media de 9,59±5,39cm (1,68±1,51 SDS) respecto a su pronóstico de crecimiento previo al tratamiento.
ConclusionesEl presente estudio evidencia como factores predictivos de buena respuesta al tratamiento con rhGH a largo plazo en orden de importancia: A) dosis de rhGH al inicio del tratamiento, B) tiempo de tratamiento con rhGH hasta inicio de terapia estrogénica, C) incremento en los niveles de IGF1 e IGFBP-3 durante el primer año de tratamiento y D) velocidad de crecimiento en el primer año de tratamiento.
Turner syndrome is a chromosomal disorder that occurs in one of every 2500 live births, characterised by the complete or partial absence of a chromosome X (the complete monosomy [45,X] accounts for more than 50% of cases),1,2 although some studies have found evidence of a high prevalence of mosaicism.3 It is associated with a range of phenotypic characteristics, chief of which are short stature, gonadal dysgenesis, hand and foot lymphoedema, pterygium colli, cubitus valgus and cardiovascular malformations, among others.2
Since short stature is a common feature and the sole clinical manifestation in most cases,1–3 several studies have evinced the efficacy of treatment with recombinant human growth hormone (rhGH), with increases in final height of ∼7–10cm.4–6
Treatment with rhGH has to be initiated early when stature is more than two standard deviations (−2 SDS) below that of the general population or height velocity (HV) is below the tenth percentile for the patient's bone age. It should not be delayed past age 4 years nor initiated before age 2 years.1,4
Different studies have identified some predictors of adult height, such as height at initiation of treatment with rhGH, response in the first year of treatment, genetic height potential, age at initiation of treatment or mean weekly dose of rhGH.4,6,7
We present a study conducted in a Spanish cohort of patients with Turner syndrome with the aim of analysing the association between the response to treatment with rhGH and various factors.
Materials and methodsWe conducted a retrospective study of 25 patients with a Turner syndrome diagnosis, 17 of who were treated with rhGH and followed up at the paediatric endocrinology unit of a tertiary level hospital until they reached their adult height. The patients treated with rhGH have been in followup from 1995 to present, and having reached adult height was an inclusion criterion. We also retrieved data for older cases that were not treated with rhGH on account of being diagnosed at older ages or the family refusing the treatment.
We reviewed the medical records of patients with Turner syndrome, collecting data for auxological measurements, laboratory tests, karyotyping and pharmacological treatment throughout their followup in the unit. We informed the patients of the purpose of the study and obtained their informed consent.
We assessed the short-term response to treatment with rhGH (12 months) based on changes in HV. We defined response to treatment as an increase in HV of more than three centimetres per year compared to the previous HV or an increase by three SDS at 12 months of treatment. To assess the long-term response to treatment (up to reaching adult height), we used five possible response variables: (1) an increase in height SDS compared to baseline SDS (delta HtSDS: adult height SDS− height at initiation of rhGH)3; (2) increase in height SDS compared to the baseline predicted adult height SDS (adult height SDS− 10th percentile SDS); (3) increase in height SDS compared to the height at initiation of oestrogen therapy as a response variable (adult height SDS− height at oestrogen initiation); (4) increase in height SDS compared to the difference of the height at initiation of treatment with rhGH and the height at initiation of oestrogen therapy (oestrogen initiation height SDS− rhGH initiation height SDS); (5) increase in height SDS relative to the duration of rhGH therapy prior to initiation of oestrogen therapy.
We conducted a descriptive and inferential analysis with SPSS 18.0 for Windows using nonparametric tests: the Wilcoxon test for quantitative variables, Spearman's correlation test for analysing the linear correlation between quantitative variables, linear regression analysis for quantitative variables that had shown a linear correlation and for quantitative and qualitative variables; the Mann–Whitney U test to compare dichotomous qualitative variables with quantitative variables, and the Kruskal–Wallis test to compare nominal qualitative variables with quantitative variables. We defined statistical significance for all tests as a p-value of less than .05.
ResultsOf the 25 patients under study, 17 (68%) received rhGH treatment, while eight did not.
The most frequent karyotype in these patients was 45,X (42.9%) followed by isochromosome 46,X,i(Xq) (17.8%) and 46,X,i(Xq)/45,X mosaicism (14.3%). Other less frequent karyotypes found in the sample were mosaicisms such as 46,XX(r)/45,X; 47,XXX/45,X/46,XX; 45,X/46,X,der(X) and 45,X/47,XXX; 46,X,i/45,X/46,XX.
When it came to phenotypic expression, we found that the classical presentation (short stature, pterygium colli, cubitus valgus, widely spaced nipples, etc.) was the most frequent phenotype (50%); while six patients did not have characteristic features (27.3%) and received the diagnosis when they underwent evaluation of short stature.
The mean age at initiation of rhGH therapy was 7.90±4.13 years (range, 1.82–16.45 years) with a mean height SDS of −2.49±0.63.
The mean initial dose of rhGH used for treatment was 0.048±0.01mg/kg/day and the mean duration of treatment was 7.36±3.88 years. At the time of treatment completion, patients had a mean age of 14.52±1.69 years and had reached a mean final adult height of 156.15±3.66cm (−1.23±0.62 SDS).
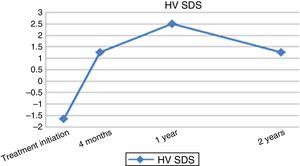
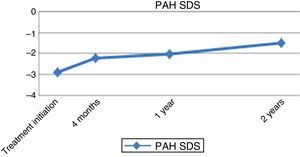
At 12 months of treatment with rhGH, there was an increased HV of 8.21±2.22cm/year (2.5±2.55 SDS) (Fig. 1). At this time, the predicted adult height for the patients had increased by 4.39±3.14cm (144.98±7.99 vs 149.37±5.92cm) and 0.67±1.06 SDS (−2.92±1.21 vs −2.24±0.91 SDS) (Fig. 2). At two years of treatment, the mean predicted adult height increased to 150.56±5.17cm, with a slight decrease in HV to 6.73±1.63cm/year.
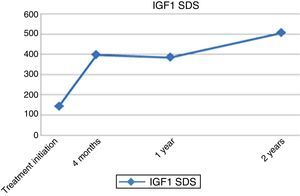
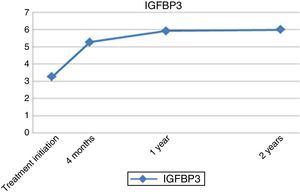
As for laboratory parameters, there was a progressive increase in the levels of IGF1 and IGFBP3 until the end of treatment, with levels reaching up to three times their baseline values, but always remaining within normal ranges (Figs. 3 and 4).
The mean final height gain compared to baseline height at initiation of treatment was of 42.82±21.23cm (total gain of 1.25±0.76 SDS). When it came to the baseline predicted adult height, height gain in patients exceeded it by a mean of 9.59±5.39cm (1.68±1.51 SDS) (Table 1).
Descriptive analysis of the long-term response to treatment with rhGH.
| Initiation of treatment | 2 years of treatment | Adult age | Total gain | |
|---|---|---|---|---|
| Height (cm) | 113.32±22.98 | 124.18±17.74 | 156.15±3.66 | 42.82±21.23 |
| Height SDS | −2.49±0.63 | −1.93±0.85 | −1.23±0.62 | 1.25±0.76 |
| Predicted adult height (cm) | 144.9±7.89 | 150.57±5.17 | 9.59±5.39 | |
| Predicted adult height SDS | −2.92±1.21 | −2.05±0.81 | 1.68±1.51 |
After 12 months of treatment, patients had improvements in height (P=.011), HV (P=.004) and IGF1 levels (P=.043) (Table 2), and we also observed an improvement in the adult heights achieved (P=.028).
Analysis of the changes in studied parameters from baseline to one year after initiation of rhGH therapy.
| At treatment initiation, mean±SDS | At one year of treatment, mean±SDS | P | |
|---|---|---|---|
| Height SDS | −2.49±0.63 | −2.09±0.80 | .028 |
| Height velocity SDS | −1.64±2.58 | 2.50±2.55 | .004 |
| Predicted adult height SDS | −2.92±1.21 | − 2.24±0.90 | .075 |
| IGF1 | 143.12±101.02 | 384.83±111.47 | .043 |
| IGFBP3 | 3.25±1.36 | 5.77±1.47 | .068 |
Our assessment of the short-term response to treatment based on increases in HV showed that in 66.6% of the patients, HV increased by more than 3cm/year at 12 months of treatment compared to baseline. Furthermore, we found a positive correlation between response to treatment and the dose of rhGH (P=.037; β=69.95), and response to treatment and increased levels of IGF1 and IGFBP3 (P=.000; r2 [IGF1]=0.846, r2 [IGFBP3]=0.809). When we analysed height gain relative to the interval between initiation of treatment with rhGH and initiation of oestrogen therapy, we found a positive correlation for auxological parameters such as height and HV both in cm and SDS at four months and one year of treatment (Table 3).
Correlation between height gain parameters and duration of treatment with rhGH before initiation of oestrogen therapy.
| P | R2 | R | β coefficient | |
|---|---|---|---|---|
| Increase in height velocity at 1 year (cm) | .094 | 0.75 | 0.86 | 0.134 |
| Increase in height velocity SDS at 1 year | .014 | 0.813 | 0.91 | 0.189 |
| Predicted adult height at rhGH initiation | .000 | 0.546 | 0.74 | 0.628 |
| Height gain SDS at 4 months | .050 | 0.372 | 0.61 | 0.685 |
| rhGH dose | .037 | 0.795 | 0.891 | 69.52 |
The mean difference in final height between patients treated with rhGH and patients not treated with it was 10.69±2.63cm (P=.031; 156.15±3.66cm vs 145.44±3.69cm, respectively).
Furthermore, we computed linear regression curves using the predictors for a favourable response identified in previous steps, but since the sample size was small, the results we obtained do not seem to be generalisable.
We did not find any differences in response to treatment based on karyotype (P=.147).
DiscussionShort stature is the key feature of Turner syndrome and may occur in the absence of other clinical manifestations.3 Linear growth deceleration starts in infancy and early childhood, and becomes more marked in late childhood and adolescence, resulting in a significantly short stature in adulthood.2 The indication of rhGH therapy to increase HV and adult height in patients with Turner syndrome is accepted and supported by various studies.1,2,4 There is evidence that this treatment results in increases of five to ten centimetres in the final adult height.4,5 Treatment should be initiated when the patient's height is two SDS below the population mean or when HV is below the tenth percentile for the patient's bone age.4,8
Several studies such as the one carried out by the Canadian Growth Hormone Advisory Committee9,10 and a few conducted in Spain have demonstrated the importance of rhGH treatment in these patients, as they have found increases in HV of 4.1–8.15cm at 12 months of treatment.11–13 There is great variability in the increases in HV reported in the literature, as they range from −1.69 to 1.97 SDS. The increase is greatest in the first year of treatment, and less significant in subsequent year.14 In our study, at 12 months of treatment we found an increased HV of 8.21±2.22cm/year (2.5±2.55 SDS) with a difference compared to baseline of 3.22cm, which corresponds to a good initial increase in HV compared to what has been reported in previous studies.11–14
In addition, research in recent years has attempted to both assess response to treatment and determine the factors that promote a good response. Thus, for the purpose of assessing response to treatment in the short term, Quigley et al.15 noted the increase in HV by ∼2cm a year. In our study, we assessed response to treatment with rhGH applying a threshold of 3cm a year for the increase in HV at 12 months of treatment, which was exceeded by 66.6% of the patients under study. Other authors16 highlight a mean increase in height SDS in the first year of treatment of +0.50±0.03 SDS. In the short term, these parameters function as predictors of a long-term favourable response to treatment, although this remains controversial.17 In the study that we present here, we observed that when it came to the longitudinal changes in the main auxological parameters, the largest changes in height, HV and predicted adult height took place in the first year (4–12 months from initiation), followed by lesser increases and an eventual plateau, except in HV.
As to the findings concerning long-term response to treatment, Radetti et al. found increases in final height of up to 9.2cm, corroborated by more recent studies showing increases in final height of ∼7cm18 and of more than 1 SDS.19,20 Our findings were similar, with a mean increase in final height compared to baseline height of 9.59±5.39cm (1.68±1.51 SDS).
There is disagreement regarding the association between the dosage of rhGH and the improvement in clinical outcomes due to increased levels of growth factors; some authors have found such an association,16,20–22 while others, including García et al.23 or Wetterau et al.,24 believe that it has no impact on the final response to treatment. In our study, we found a positive correlation with the increase of growth factor levels (IGF1 and IGFBP3) at one year of treatment, which is strongly associated with the administered dose of rhGH and better clinical outcomes.14,25 Furthermore, studies conducted in recent years have attempted to identify the genes involved in the early response of these patients to rhGH. Some have identified polymorphisms in the CDK4 gene that may have an impact on IGF1 levels and therefore on the response to treatment with rhGH.26 Ranke et al.4,25,27 found positive correlations with the administered dose (greater than 0.27mg/kg/day), frequency (more days per week) and age at initiation (some authors assert that adult height can reach the normal range if treatment is initiated between 2 and 4 years of age6,19), with no consensus on the subject.28
The duration of treatment with rhGH also seems to influence the final height gain. Chernausek et al.19,22,27 described this time interval as an important predictor of final height, and even produced an equation to estimate the approximate height gain: height gain (in cm=2.1× years of rhGH treatment prior to oestrogen initiation.
The timing of initiation of oestrogen therapy continues to be debated; some authors27–29 have found greater height gains with initiation of oestrogen therapy at age 14 years; other studies suggest that low doses of oestrogen administered at earlier ages may lead to an increase of 0.37 SDS in adult height compared to patients in whom initiation of oestrogen therapy is delayed.29 In our opinion, the timing of oestrogen initiation should be decided in agreement with the patient and the family, taking into account not only auxological parameters such as height or bone age, but also bone mineral density and the wishes expressed by the patient in relation to treatment initiation and pubertal development.
Another controversial aspect is the use of anabolic steroids such as oxandrolone, as it seems to increase final height when combined with rhGH. Gault et al.28 found a mean increase in final height of +4.6cm (range, 1.9–7.2cm) in a group treated with oxandrolone compared to a group treated with placebo. However, their study did not find a statistically significant positive additive effect for the combination of oxandrolone and late pubertal induction.
Our comparison of patients treated with rhGH and patients not treated with rhGH was consistent with the findings of Paschino et al.30 and Rosenfeld et al.,31 with a mean difference in height between both groups of 10.69±2.63cm (P=.031), which evinces the importance of early treatment in these patients. Ranke et al.18 studied the possibility of patients with specific karyotypes responding better to treatment with rhGH, but we found no differences based on karyotype.
Based on the results of our study, we determined that the predictors for a long-term favourable response are, from most important to least: (A) dose of rhGH at treatment initiation, (B) duration of treatment with rhGH prior to initiation of oestrogen therapy, (C) increased levels of IGF1 and IGFBP3 during the first year of treatment and (D) HV in the first year of treatment.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez Marco SB, de Arriba Muñoz A, Ferrer Lozano M, Labarta Aizpún JI, Garagorri Otero JM. Hormona de crecimiento y síndrome de Turner. An Pediatr (Barc). 2017;86:81–86.