To analyse the effectiveness of growth hormone (GH) therapy in short-stature children born small for gestational age (SGA) without catch-up growth (height at the beginning of treatment<−2.5 SDS), in Valencia (Spain), between 01/01/2003 and 12/31/2013; and to compare our findings with previously published data.
Materials and methodsAnthropometric data from the SGA children were obtained from the database of the “Ministry of Health of Valencia”. These data were retrospectively reviewed.
ResultsA total of 115 SGA children, with a mean age of 8.10±2.75 years and height of −3.14±0.59 SDS started treatment (dose: 0.035±0.004mg/kg/day) between January 1st, 2003 and March 31st, 2013. After 2 years of therapy (n=115, age: 10.50±2.72 years) the height SDS was −2.11±0.66; and after 4 years (n=96, age: 12.65±2.46 years) of −1.76±0.75 SDS. This latest improvement in stature matches ages at which the growth spurt usually occurs. Only 35 out of 115 children reached adult height, although impaired (−2.22±0.86 SDS), and failed to achieve their target height (−1.72±0.75 SDS). However, this sub-group grew to near the height of the shorter parent (−1.95±1.28 SDS), and 42.9% of these 35 cases increased their stature by more than 1 SDS.
ConclusionsThe studied sample did not achieve satisfactory growth results, as in other published series. Our findings might be improved by starting treatment earlier, and with doses individualised according to patient characteristics.
Valorar la efectividad del tratamiento con hormona de crecimiento (GH) en niños pequeños para la edad gestacional (PEG) sin crecimiento recuperador (talla al iniciar la terapia<−2,5 DE), en la Comunidad Valenciana, entre el 01/01/2003 y el 31/12/2013, y comparar los resultados con los ya publicados.
Material y métodosLos datos antropométricos de los PEG que constituyeron la muestra de estudio se recogieron retrospectivamente de los documentos de solicitud de tratamiento existentes en la Consejería de Sanidad de Valencia.
ResultadosUn total de 115 sujetos comenzaron a tratarse entre el 01/01/2003 y el 31/12/2013, con dosis de GH de 0,035±0,004mg/kg/día, a una edad de 8,10±2,75 años y con una talla de −3,14±0,59 DE. Talla alcanzada tras 2 años de terapia (n=115, edad: 10,50±2,72 años): −2,11±0,66 DE; y tras 4 años (n=96, edad: 12,65±2,46 años): −1,76±0,75 DE. Esta última mejoría de talla coincide con edades a las que suele producirse el estirón puberal. Solo 35 de los 115 niños finalizaron el crecimiento, en el periodo de estudio, a una edad de 16,22±1,19 años. Este subgrupo no consiguió normalizar la talla adulta (−2,22±0,86 DE), ni alcanzar su talla diana (−1,72±0,75 DE); no obstante, hubo una buena aproximación a la talla del progenitor más bajo (−1,95±1,28 DE). El 42,9% de estos 35 casos experimentaron un incremento de estatura superior a 1 DE.
ConclusionesLa muestra estudiada no obtiene una respuesta de crecimiento tan satisfactoria como las de otras series publicadas. Probablemente, estos resultados mejorarían iniciando el tratamiento más precozmente, e individualizando las dosis según las características del paciente.
Small for gestational age (SGA) refers to newborns whose birth weight or length are 2 or more standard deviations (SDs) below the mean for their sex and gestational age in the reference population.1 This results from abnormalities in intrauterine growth, whose cause is unknown in 40% of cases.2
Approximately 90% of these children exhibit spontaneous catch-up growth in the first two years of life, although this may be delayed until age 3–4 years in children born preterm.1 This catch-up growth does not occur in the remaining 10%, in most cases for unknown reasons.3,4 Some of the possible causes that have been proposed include a decreased cell mass at the time of birth and alterations of endocrine-metabolic pathways involved in postnatal growth, especially in the GH-IGF-1 axis. However, growth hormone stimulation tests show normal or even elevated levels of growth hormone (GH) in most SGA children,5 which suggests some degree of peripheral resistance to GH activity or a reduced biological activity of GH.
Job and colleagues were the first to use recombinant human growth hormone (rhGH) for the treatment of delayed postnatal growth seen in some children born SGA,6,7 and set the foundations for subsequent research, the results of which suggests that rhGH is a safe and effective treatment to partially reduce stature deficits in the adult height of individuals born SGA. However, the response to treatment varies widely between studies, which may be related to the diverse aetiology of SGA.8–11
The use of this hormone for the treatment of short children born SGA was authorised by the Food and Drug Administration (FDA) in July 2001 and by the European Agency for the Evaluation of Medicinal Products (EMA) in June 2003.1 The Spanish Agency of Medicines and Health Care Products (Agencia Española del Medicamento y Productos Sanitarios [AEMPS]) follows the criteria set by the EMA.
The dosage of rhGH recommended for children born SGA is higher than the one in children with GH deficiency, and ranges between 0.035 and 0.067mg/kg/day.1 The reason for this is that, as noted above, SGA patients exhibit a degree of peripheral resistance to GH activity rather than decreased GH release.
No studies have been conducted on the effectiveness of treatment in individuals born SGA in the Autonomous Community of Valencia since this indication for rhGH was approved. In this article, we present the growth outcomes in a sample of children treated over a 10-year period.
Objectives- (a)
To assess the effectiveness of growth hormone in children born SGA in our area, between January 01, 2003 and December 31, 2013, based on the proportion of these patients that reached their target height by the end of treatment, or the corresponding growth trajectory if treatment was not completed.
- (b)
To compare the obtained results with those published by other research groups in Spain and abroad.
We used the information gathered in the request forms for initiation, renewal and completion of rhGH therapy submitted to the Growth Hormone Assessment Committee of the Autonomous Community of Valencia by every paediatric endocrinology unit in the hospitals of the three provinces between January 1, 2003 and December 31, 2013.
We obtained permission to access these data from the Clinical Research Ethics Committee of the Fundación de Investigación of the Hospital Clínico Universitario de Valencia. The study was classified as an observational post-authorisation study—other designs (OPA-OD) based on the directives of Order SAS/3470/2009, of December 16 of the Ministry of Health. These are retrospective studies in which due to the size of the sample or the long time elapsed since data collection, the informed consent of included individuals is not required, as long as their anonymity is safeguarded. Furthermore, the study was approved by the Department of Health of the Autonomous Community of Valencia, as these documents are stored for safekeeping in its facilities, where we carried out data collection.
We obtained the study sample applying the following criteria:
- (a)
Inclusion: we only included children born SGA that strictly fulfilled all the following prerequisites:
- -
Birth weight or length of less than −2 SDS.
- -
Height from birth consistently below −2.5 SDS.
- -
Had not reached puberty.
- -
Had received treatment with growth hormone for a minimum of two years.
- -
- (b)
Exclusion: of all children that met the inclusion criteria, we excluded those whose target height was unknown and those in whom treatment was discontinued prematurely due to the development of adverse reactions or poor adherence.
We studied the following variables retrospectively:
- 1.
Prescribed dosage of GH (mg/kg/day).
- 2.
Family anthropometric data: target height (TH) SDS and height SDS of the shortest parent.
- 3.
Patient anthropometric data: decimal age in years, SDS for height and growth velocity (GV). Specifically, these data were collected at five phases: 1st, prior to starting treatment; 2nd, after one year of treatment; 3rd, after two years of treatment; 4th, the last year they received treatment; and 5th, adult height.
We found the SDS of the aforementioned anthropometric measures by applying the tables of the 2010 Spanish Cross-sectional Growth Study12,13 (TH, height of the shortest parent and patient heights in the five phases of the study) and of the 1978–2000 Spanish LongitudinalStudy14 (for GV, keeping into account the maturation stage of the patient).
We considered that patients had reached their adult height when their grow rate became less than 2cm per year or their growth plates had closed.
Statistical methodsWe have described the baseline characteristics of our sample using the mean and standard deviation. To assess the effectiveness of treatment, we compared the mean height achieved in each phase of the study with the height of the phase immediately preceding it using Student's t test for paired samples with the Bonferroni correction. We used Student's t test for independent samples to compare the mean adult height with the TH (both variables followed a normal distribution) and the Mann–Whitney U test to compare the mean adult height with the height of the shortest parent (in this case, we used a nonparametric test because the distribution of the standard deviation scores [SDS] for the height of the shortest parent was not normal).
We considered the results statistically significant when the p-value was less than .05. We performed the statistical calculations with SPSS version 20®.
ResultsA total of 1361 requests for GH treatment were submitted to the Assessment Committee of the Autonomous Community of Valencia during the period under study, but only 134 met the inclusion criteria. The remaining 1216 corresponded to requests that were denied because they did not meet the established criteria for SGA or to requests for adult or paediatric patients with indications different than the one under study (GH deficiency, Turner syndrome, Prader-Willi syndrome, renal failure, SHOX mutations).
Exclusion criteria applied to 19 of the 134 cases. In 14 out of the 19, there was no record of the mean parental height. In two, there was clear documentation of non-adherence by the family: the prescribing physician personally informed the Committee of the discontinuation of the treatment. In three, treatment was discontinued due to adverse events that were attributed to GH treatment: one patient with left-side slipped capital femoral epiphysiolysis that required surgery, one patient with Perthes disease, and one patient with hyperglycaemia of several months’ duration. Therefore, the final sample consisted of 115, 60 male and 55 female, all of them Caucasian.
The mean administered dose of GH was 0.035±0.004mg/kg/day. The 115 patients received treatment for a minimum of two years (as was required for inclusion in the study), and only 96 of the 115 reached the fourth phase (three or more years of treatment). Information on the adult height was only available for 35 of the 115 patients.
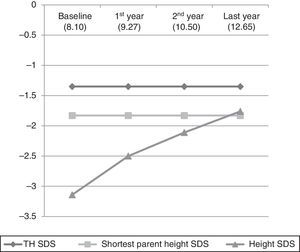
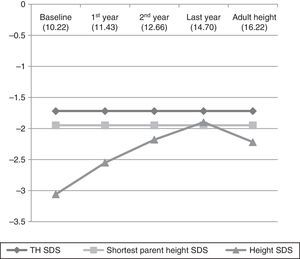
Table 1 and Fig. 1 show the evolution of height and GV in the patients that did not reach their final height. Table 2 and Fig. 2 show the evolution of the 35 children that reached their final height during the period under study.
Anthropometric data of the sample throughout the study (mean±standard deviation).
| Family anthropometric data | N=115 |
| Target height SDS | −1.35±0.80 |
| Height SDS of the shortest parent | −1.83±0.83 |
| Before treatment initiation | N=115 |
| Age (years) | 8.10±2.75 |
| Height SDS | −3.14±0.59 |
| After one year of treatment | N=115 |
| Age (years) | 9.27±2.73 |
| Height SDS | −2.50±0.63 |
| Growth velocity SDS | 2.12±2.01 |
| After two years of treatment | N=115 |
| Age (years) | 10.50±2.72 |
| Height SDS | −2.11±0.66 |
| Growth velocity SDS | 1.25±1.40 |
| Last year of treatment | N=96 |
| Age (years) | 12.65±2.46 |
| Height SDS | −1.76±0.75 |
| Growth velocity SDS | 0.48±1.35 |
Graphic representation of the evolution of the mean height standard deviation scores in the first four phases of the study. The numbers in parentheses correspond to the mean chronological age in each phase of treatment. Initial sample (115 patients), first year (115 patients), second year (115 patients), last year (96 patients).
Anthropometric data of the 35 patients that reached the target height (mean±standard deviation).
| Family anthropometric data | |
| Target height SDS | −1.72±0.75 |
| Height SDS of the shortest parent | −1.95±1.28 |
| Before treatment initiation | |
| Age (years) | 10.22±1.87 |
| Height SDS | −3.06±0.62 |
| After one year of treatment | |
| Age (years) | 11.43±1.87 |
| Height SDS | −2.55±0.69 |
| Growth velocity SDS | 1.48±1.63 |
| After two years of treatment | |
| Age (years) | 12.66±1.92 |
| Height SDS | −2.18±0.74 |
| Growth velocity SDS | 0.85±0.99 |
| Last year of treatment | |
| Age (years) | 14.71±1.41 |
| Height SDS | −1.90±0.80 |
| Growth velocity SDS | 0.43±1.34 |
| Data on final height | |
| Chronological age at which adult height was reached (years) | 16.22±1.19 |
| Adult height SDS | −2.22±0.86 |
| Δ (adult height SDS−initial height SDS) | 0.85±0.67 |
| Δ (adult height SDS−target height SDS) | −0.49±0.71 |
| Δ (adult height SDS−shortest parent height SDS) | −0.27±1.55 |
We found a statistically significant increase in height from treatment initiation to the fourth phase of the study (P<.0001). As seen in Fig. 1, the mean height of the shortest parent was reached and even surpassed, and there seems to be a trend towards approximating the TH. When we did a separate analysis of the 35 patients that reached their adult height, we found that their evolution in the first four phases was the same as the evolution in the rest of the sample, that is, they had a statistically significant increase in height (P<.0001), but starting in the fourth phase there was a marked decrease in growth and the adult height that was ultimately achieved was short (SDS, −2.22±0.86). When we compared their adult height with their TH (SDS, −1.72±0.75), the difference was statistically significant (Student's t, −2.545; P=.013), but when we compared the adult height achieved with the height of the shortest parent (SDS, −1.95±1.28) the difference was not statistically significant (Mann–Whitney U test z score, −0.582; P=.561).
Of the 35 patients that reached their adult height, 42.9% experienced an increase of more than one SDS in height with GH treatment.
DiscussionThis is the first study that presents growth outcomes in Valencian children born SGA treated with GH.
Although the protocol of our autonomous community does not allow GH treatment in individuals with certain syndromes (Silver Russell, Noonan, Down), our sample was still not homogeneous, as treatment protocols do not guarantee the exclusion of children with syndromes or with certain bone dysplasias (especially those that are not clinically significant in the first years of life). However, the aim of this study was to assess the overall response to GH treatment of patients classified as SGA. It is important to note that we studied the data of treatment request documents as opposed to actual patients. Therefore, we were only aware of patients having abnormal phenotypes if the physician that put in the request for GH treatment documented it (these cases were excluded from the study). The heterogeneity of the sample may have influenced the results. In future prospective studies on this subject, it may be useful to document the presence of syndromic features as well as the measurements of body segments in children born SGA prior to treatment initiation, as opposed to height alone.
It would be fair to say that the children in our sample achieved a statistically significant increase in their height SDS in the first four phases of the study (P<.0001). However, we found a substantial deceleration of starting in the fourth phase in the subset of 35 patients that reached their adult height, which was short (SDS, −2.22±0.86). Therefore, contrary to the outcomes reported in other series,15–17 the patients born SGA in our sample that reached their adult height did not reach normal heights nor approximate their TH. Nevertheless, they succeeded in approximating the height SDS of the shortest parent. None of the studies that we reviewed had considered the assessment of adult height in relation to the height of the shortest parent. We believed that such a comparison would be of interest in our study, considering the suboptimal adult height outcomes that we found.
We analysed the factors that may have influenced the poor growth outcomes achieved in Valencian children born SGA. According to the models for the prediction of the response to rhGH in children born SGA published by Ranke et al.,18,19 the factors that correlate most strongly with growth during the first year of treatment are the age of initiation and the GH dose, while in the second year, the most relevant factor was the GV achieved during the first year. All of these factors were unfavourable in our sample.
In our case series, children started treatment at an older age than children in other studies that had better outcomes15–17,20 (Table 3). The age at the start of treatment was particularly high in the 35 patients that reached their final heights (10.22±1.87 years). The literature emphasises the importance of initiating treatment as early as possible to achieve good outcomes16,21,22; in fact, it seems that this is one of the most relevant factors.9,17,19,23One possible reason for the delayed initiation in our study is that the sample includes patients that were already older when the indication for treatment was approved in Spain in 2003. Another possible reason, which may have had a greater weight, is the delayed referral of children from primary care to endocrinology clinic.
Comparison of results obtained in our sample of SGA children with those of other studies.
| Data prior to initiation of treatment with rhGH | KIGS20 (n=65) Median (P10/P90) | Van Pareren et al.17 (n=28) | Carrascosa et al.15 (n=49) | Güemes-Hidalgo et al.16 (n=52) | Our sample (n=115) |
|---|---|---|---|---|---|
| Chronological age (years) | 7.9 (3.6/11.9) | X±SD 7.9±1.9 | X±SD H: 7.8±1.8 M: 6.5±1.4 | X±SD 5.90±2.29 | X±SD 8.10±2.75 |
| Height SDS | (Prader) −3.6 (−5.0/−2.6) | (Prader) −2.9±0.8 | −3.3±0.6 | −3.12±0.62 | −3.14±0.59 |
| Prescribed rhGH dosage (mg/kg/day) | 0.035 (0.022/0.061) | X: 0.033 | 0.0319±0.0035 | Range: 0.033–0.045 | 0.035±0.004 |
| After 1 year of treatment | KIGS20 (n=65) Median (P10/P90) | Van Pareren et al.17 (n=28) | Carrascosa et al.15 (n=49) | Güemes-Hidalgo et al.16 (n=45) | Our sample (n=115) |
|---|---|---|---|---|---|
| Height SDS | (Prader) −3.0 (4.6/−2.0) | Not documented | Δheight SDS in 1 year: 0.8±0.3 | X: −2.2 P5: −2.4 P95: −1.9 | X±SD: −2.50±0.63 |
| Growth velocity SDS | (cm/year) 7.5 (5.6/10.0) | Not documented | X±SD 4.5±1.8 | X: 3.22 P5: 2.59 P95: 3.85 | X±SD: 2.12±2.01 |
| After two years of treatment | KIGS20 (n=65) Median (P10/P90) | Van Pareren et al.17 (n=28) | Carrascosa et al.15 (n=49) | Güemes-Hidalgo et al.16 (n=36) | Our sample (n=115) |
|---|---|---|---|---|---|
| Height SDS | Not documented | X±SD: −1.5±0.7 | Δheight SDS in 2 years: 1.2±0.4 | X: −1.8 P5: −2.0 P95: −1.5 | X±SD: −2.11±0.66 |
| Growth velocity SDS | Not documented | Not documented | X±SD: 2.0±1.4 | X: 2.28 P5: 1.7 P95: 2.87 | X±SD: 1.25±0.66 |
| Adult height | KIGS20 (n=65) Median (P10/P90) | Van Pareren et al.17 (n=28) | Carrascosa et al.15 (n=49) X±SD | Güemes-Hidalgo et al.16 | Our sample (n=35) X±SD |
|---|---|---|---|---|---|
| Chronological age (years) | 16.8 (14.7/18.3) | X: 15.8 | Male: 17.4±0.8 Female: 15.4±0.8 | – | 16.22±1.19 |
| Height SDS | (Prader) −2.1 (−3.5/−1.3) | X±SD: −1.1±0.8 | −1.7±0.7 | – | −2.22±0.86 |
| Δ (adult height SDS−initial height SDS) | (Prader) 1.3 (0.2/2.6) | X: 1.8 | 1.6±0.8 | – | 0.85±0.67 |
| Δ (adult height SDS−parental height SDS) | (Prader) −1.0 (2.4/0.0) | Not documented | −0.4±0.8 | – | −0.49±0.71 |
| Δ (adult height SDS−shortest parent height SDS) | Not documented | Not documented | Not documented | – | −0.27±1.55 |
SD, standard deviation; SDS, standard deviation score; X, mean.
The mean dose of rhGH administered to the patients in our study (0.035±0.004mg/kg/day) was similar to the dose given in other samples15–17,20 (Table 3), at the lower end of the recommended range for individuals born SGA.1,24 There is evidence that the higher the dose used—within the therapeutic ranges—the better the outcomes achieved.1,25,26 Still, if treatment is initiated at 4–6 years of age with 0.033mg/kg/day, the response is very good. However, prepubertal children with extremely short stature (below −3 SDS)and children that start treatment when they are approaching puberty may require higher doses of 0.055mg/kg/day or more.27,28 Children in either of these two categories were included in the subset of 35 cases that reached their final height, as treatment in this subset started at a mean age of 10.22 years with a mean height SDS of −3.06. The height outcomes in this group may have been better if they had been treated with doses in the upper recommended range.
The growth velocity SDS achieved by the children born SGA in our sample in the first twelve months of treatment was clearly inferior to the one achieved by other groups15–17,20 (Table 3). It is fair to assume that this outcome was influenced by the factors previously discussed (late age at initiation of treatment and suboptimal rhGH dosage).
The truly relevant data in our study corresponds to the subset of 35 patients that reached their adult height. Nevertheless, we present the evolution of the stature of the rest of the patients to underscore that it was very similar to that of those 35 subjects up to the fourth phase. In other words, the charts in Figs. 1 and 2 are practically superimposable. We believe that this is worth highlighting, as the mean age of the patients in the fourth stage (12.65 years) probably coincides with the timing of the growth spurt, and therefore, the improvement in the height SDS seen in this phase (SDS, −1.76±0.75) may be due to this event rather than to treatment. This may account for the decrease in the height SDS of the 35 patients that reached their final height relative to the previous phase, with an abrupt halt in growth following the pubertal growth spurt. We expect that the final adult height of the patients that have yet to reach it will be similar to that of the patients that already have. However, we cannot be certain until the final adult height data is available for the 115 cases.
ConclusionsThe growth outcomes of the studied sample of Valencian children born SGA treated with rhGH for short stature were not as satisfactory as those reported for other published case series. Although their height SDS did improve, they failed to approach their TH and remained with a SDS similar to that of their shortest parent. We believe that these suboptimal results were due to the advanced age at which these children started treatment, and to not having increased the dosage of rhGH taking into account the considerably delayed start, as is recommended in the literature. In order to improve these outcomes, we think it is essential that primary care paediatricians refer children born SGA that have a height SDS of less than −2.5 at around 4 years of age for prompt initiation of treatment, and that the dosage be customised based on the characteristics of the patients.
Conflict of interestThe authors have no conflict of interest to declare.
We want to thank the entire staff of the Conselleria de Sanitat, and especially Carmen Albeldaand María Antonia Grau. We also want to thank the physicians in all the hospitals of the Autonomous Community of Valencia that submitted the request forms for treatment with growth hormone to the Conselleria de Sanitat, as these were the source of the data for this study.
Please cite this article as: Sánchez Zahonero J, López García MJ. Estudio valenciano sobre tratamiento con hormona de crecimiento en pequeños para la edad gestacional. An Pediatr (Barc). 2017;86:87–93.