There is not much information about the care of infants with hypoxic-ischaemic encephalopathy (HIE) treated with therapeutic hypothermia (TH) in Spain. This includes whether protocols are routinely used, the type of neuro-monitoring performed, and how information on the neurological prognosis is presented to families. The answers to these would allow to detect and implement areas of improvement.
MethodA cross-sectional analysis was performed on the responses to structured questionnaires sent to all the Spanish neonatal units that were performing TH in June 2015. Questions were divided into 5 sections: (1) the availability of protocols and technological resources, (2) the use of neuro-monitoring tools, (3) the knowledge and training of the professionals; (4) the prognostic information given to the parents; and (5) the discharge report and the follow-up plan.
ResultsMost centres (95%) use servo controlled whole-body cooling methods and have specific management protocols. Sedation is used in 70% of centres, and in 68% of them the onset of enteral feeding is delayed until the end of the cooling period. Amplitude-integrated electroencephalography monitoring is used in more than 80% of the centres, although only in 50% are nurses able to interpret it. Cerebral oxygen saturation is not often monitored (16%). As regards diagnostic-prognostic studies, neuroimaging is universal, but brain damage biomarkers are hardly used (29%). Prognostic information is offered within the first 72 posnatal hours in 21% of the centres, and is given without the presence of the nurse in 70% of the centres. Follow-up is performed by a neuro-paediatrician (84%), with an uneven duration between centres.
ConclusionsThe care of infants with HIE treated with TH in Spain is generally adequate, although there are areas for improvement in neuromonitoring, sedation, prognostic information, teamwork, and duration of follow-up.
Apenas conocemos cómo es la asistencia de los recién nacidos (RN) con encefalopatía hipóxico-isquémica (EHI) en hipotermia terapéutica (HT), especialmente si existen protocolos asistenciales, la neuromonitorización que se realiza o cómo es la aproximación al pronóstico neurológico. Este conocimiento permite detectar e implementar áreas de mejora asistencial.
MétodoEstudio transversal de los 57 hospitales españoles que realizaban HT en 2015, mediante cuestionario sobre: 1) la disponibilidad de protocolos y de recursos tecnológicos; 2) el uso de herramientas de neuromonitorización; 3) los conocimientos de los profesionales; 4) la información pronóstica que se da los padres, y 5) el informe al alta y del plan de seguimiento.
ResultadosEl 95% utiliza enfriamiento corporal-total servocontrolado y dispone de protocolos específicos de actuación. El 70% utiliza sedación y el 68% deja al paciente a dieta absoluta. La monitorización con electroencefalografía integrada por amplitud se utiliza en más del 80% de los centros, aunque solo en el 50% la enfermera es capaz de interpretarlo. La saturación de oxígeno cerebral es escasamente monitorizada (16%). Entre los estudios diagnóstico-pronósticos, la neuroimagen es universal, pero los neurobiomarcadores apenas se utilizan (29%). Solo el 21% ofrece información pronóstica antes de las 72 h de vida; sin presencia de la enfermera en el 70%. El seguimiento lo realiza el neuropediatra (84%), con una duración desigual entre centros.
ConclusionesLa asistencia del RN con EHI en España es adecuada, con áreas de mejora en: neuromonitorización, sedación, marco temporal de la información pronóstica, trabajo en equipo y estandarización de la duración del seguimiento.
Therapeutic hypothermia (TH) is the standard of care for managing newborns (NBs) with perinatal hypoxic-ischaemic encephalopathy (HIE). Although TH is used widely, the management of these infants must be improved to optimise the outcomes of this intervention. To this end, it is essential that protocols for the care of NBs with HIE involve a holistic approach with continuity of care from birth through the development of a post-discharge follow-up plan.1,2 Within this holistic approach, neurologic monitoring, sedation and other aspects of care during TH and the tools used to assess the extent of brain injury are relevant aspects in the management of NBs with HIE.3–5
At present, evidence is available on the implementation of TH in NBs with HIE in most European countries, but this evidence hardly offers information on how the care is actually delivered, including whether there is a care protocol, the type of neurologic monitoring used, or the approach to making a neurologic prognosis. There is also a paucity of information on the challenges faced by medical professionals in estimating the severity of injury, in informing families and on the participation in care delivery of the nursing staff. Such information would allow us to identify opportunities for improvement and to promote the necessary changes to optimise the holistic management of NBs with HIE.6
With the aim of understanding the current situation in the management of NBs with HIE and identify current needs and areas of improvement, we conducted a nationwide cross-sectional study on moderate-to-severe HIE in 2015. The data concerning the incidence of HIE, adherence to the use of TH and the management of NBs with perinatal asphyxia eligible for TH in the first hours of life have been published in previous documents.7,8
The objectives of this study were: (1) assessing whether there was a protocol in place for the care of NBs with HIE from initiation of TH to the discharge home; (2) which neurologic monitoring and diagnostic tools were used to make a prognosis and how they were used and (3) identify the main needs and difficulties reported by health care providers.
MethodsWe conducted a nationwide cross-sectional study on moderate-to-severe HIE in NBs delivered at or after 35 week's gestation by means of an online survey. The study included every tertiary care hospital in Spain, public or private, with level II or III units that provided TH in June 2015 (n=57).7,8
The questionnaire comprised 119 items in 5 sections: (1) availability of protocols and technological resources (Tables 1 and 2); (2) use or neurological monitoring systems (Table 3); (3) knowledge of health care providers (Table 4); (4) aspects concerning prognosis and related information (Tables 5 and 6), and (5) aspects related to the discharge report and the followup plan (Table 7).
Items regarding the availability and use of resources in the care of newborns with HIE during TH in Spain, number of hospitals n (%).
| Nearly always | Often | Rarely or never | |
|---|---|---|---|
| Available | |||
| 1. At least 1 active cooling systema | 54 (95%) | 0 | 3 (5%) |
| 2. At least 1 aEEG system | 51 (89%) | 0 | 6 (11%) |
| 3. Psychologist to support parents-families | 38 (72%) | 6 (11%) | 9 (17%) |
| 4. Follow-up plan after discharge | 56 (100%) | 0 | 0 |
| 5. Multidisciplinary follow-up team | 40 (71%) | 9 (16%) | 7 (13%) |
| Used | |||
| 6. Routine neurologic examination | 48 (86%) | 7 (12%) | 1 (2%) |
| 7. aEEG | 47 (84%) | 1 (2%) | 8 (14%) |
| 8. Conventional electroencephalography | 54 (96%) | 2 (4%) | 0 |
| 9. Cranial MRI | 52 (93%) | 3 (5%) | 1 (2%) |
| 10. Cranial MRI from 7 days post birth | 47 (84%) | 5 (9%) | 4 (7%) |
| 11. Cranial MRI in first 3–4 days included diffusion-weighted MRI and/or spectroscopy | 11 (20%) | 13 (23%) | 32 (57%) |
| 12. Head Doppler ultrasound | 46 (82%) | 4 (7%) | 6 (11%) |
| 13. Regional cerebral oxygen saturation | 9 (16%) | 7 (13%) | 40 (71%) |
| 14. Neurologic markers in CSF or blood | 16 (29%) | 6 (11%) | 34 (60%) |
aEEG, amplitude-integrated electroencephalogram; CSF, cerebrospinal fluid; HIE, perinatal hypoxic-ischaemic encephalopathy; MRI, magnetic resonance imaging; TH, therapeutic hypothermia.
Items on the protocols and interventions performed during the care of newborns with HIE undergoing therapeutic hypothermia in Spain, number of hospitals n (%).
| Nearly always | Often | Rarely or never | |
|---|---|---|---|
| The hospital has a specific protocol for | |||
| 15. Assessment and rating of severity of HIE | 49 (92%) | 2 (4%) | 2 (4%) |
| 16. Assessments to monitor systemic involvement | 49 (92%) | 3 (6%) | 1 (2%) |
| 17. Neurologic and imaging evaluations | 51 (96%) | 1 (2%) | 1 (2%) |
| 18. Neurophysiological assessment | 49 (92%) | 2 (4%) | 2 (4%) |
| 19. Administration of sedation | 44 (83%) | 7 (13%) | 2 (4%) |
| During therapeutic hypothermia | |||
| 20. The newborn receives enteral nutrition | 5 (9%) | 12 (23%) | 36 (68%) |
| 21. Parents can hold the infant | 1 (2%) | 5 (9%) | 47 (89%) |
| 22. Sedation is used in newborns with moderate HIE | 38 (72%) | 14 (26%) | 1 (2%) |
| 23. Sedation is used in newborns with severe HIE | 41 (77%) | 9 (17%) | 3 (6%) |
| 24. Sedation is given as continuous infusion | 40 (76%) | 6 (11%) | 7 (13%) |
| 25. Sedation is monitored with clinical scales | 17 (32%) | 13 (25%) | 23 (43%) |
| 26. Sedation is avoided if newborn is not intubated to prevent depression | 6 (11%) | 11 (21%) | 36 (68%) |
| 27. Fentanyl used for sedation | 36 (68%) | 15 (28%) | 2 (4%) |
| 28. Morphine or a similar opioid used for sedation | 12 (23%) | 15 (28%) | 26 (49%) |
| 29. Midazolam is used for sedation | 6 (11%) | 9 (17%) | 38 (72%) |
HIE, perinatal hypoxic-ischaemic encephalopathy.
Main tools used for assessment of brain injury in newborns with HIE managed with therapeutic hypothermia in Spain, number of hospitals n (%).
| Nearly always | Often | Rarely or never | |
|---|---|---|---|
| Neonatal neurologic examination | |||
| 30. Qualified neonatologist on staff | 40 (75%) | 12 (23%) | 3 (2%) |
| 31. Qualified paediatric neurologist on staff | 28 (50%) | 17 (30%) | 11 (20%) |
| Use of aEEG | |||
| 32. The main challenge is interpreting the background tracings | 2 (4%) | 9 (16%) | 45 (80%) |
| 33. The main challenge is identifying seizures | 6 (11%) | 24 (43%) | 26 (46%) |
| 34. Interpreted by nurse, calling on physician as needed | 9 (16%) | 19 (34%) | 28 (50%) |
| Use of conventional EEG | |||
| 35. Neonatologist trained on EEG interpretation on staff | 5 (9%) | 8 (14%) | 43 (77%) |
| 36. At least 1 EEG recording made before discharge | 53 (95%) | 3 (5%) | 0 |
| 37. Only performed in case of detection of clinical or electrical (aEEG) seizures | 3 (5%) | 2 (4%) | 51 (91%) |
| 38. If requested, it is performed within 48 h | 40 (72%) | 12 (21%) | 4 (7%) |
| Use of head ultrasound | |||
| 39. Performed by neonatologist | 20 (35%) | 15 (26%) | 22 (39%) |
| 40. Performed by radiologist | 36 (63%) | 13 (23%) | 8 (14%) |
| 41. Evaluation completed with transcranial Doppler ultrasound | 47 (82%) | 5 (9%) | 5 (9%) |
| 42. Performed in the first 6 h | 28 (49%) | 14 (25%) | 15 (26%) |
| 43. One scan a day in the first few days | 36 (63%) | 10 (18%) | 11 (19%) |
| 44. One scan before discharge | 47 (82%) | 3 (5%) | 7 (12%) |
| Use of head MRI | |||
| 45. Neonatologist trained on head MRI interpretation on staff | 7 (12%) | 20 (35%) | 30 (53%) |
| 46. Radiologist trained on head MRI interpretation on staff | 39 (68%) | 16 (28%) | 2 (4%) |
| 47. Performed at the hospital | 52 (91%) | 0 | 5 (9%) |
| 48. Patient transferred to a collaborating facility outside hospital for MRI | 4 (7%) | 0 | 52 (93%) |
| 49. Performed in every NB with moderate/severe HIE | 55 (98%) | 0 | 1 (2%) |
| 50. Performed in NBs with mild HIE | 10 (18%) | 9 (16%) | 37 (66%) |
| 51. Performed within 1 week from birth | 22 (39%) | 13 (23%) | 21 (38%) |
| 52. Only 1 scan performed between 7–28 days | 29 (52%) | 10 (18%) | 17 (30%) |
| Testing for biomarkers (NSE, S-100 protein, other) | |||
| 53. In CSF samples | 6 (11%) | 3 (5%) | 47 (84%) |
| 54. In blood samples | 6 (11%) | 0 | 50 (89%) |
| 55. In urine samples | 0 | 2 (4%) | 54 (96%) |
| 56. Performed in house | 11 (20%) | 4 (7%) | 41 (73%) |
| 57. Turnaround time < 48 ha | 8 (14%) | 5 (9%) | 2 (77%) |
aEEG, amplitude-integrated electroencephalography; CSF, cerebrospinal fluid; EEG, electroencephalography; HIE, hypoxic-ischaemic encephalopathy; NB, newborn; NSE, neuron-specific enolase.
Items regarding the knowledge of the care team on the tools used in newborns with HIE managed with TH (4 a) and the most necessary educational interventions (4 b), number of hospitals n (%).
| (a) Knowledge of unit staff on the following tools | Adequate | Little but sufficient | Clearly insufficient |
|---|---|---|---|
| 58. Interpretation of neurologic examination | 45 (81%) | 8 (14%) | 3 (5%) |
| 59. Amplitude-integrated electroencephalography | 38 (68%) | 15 (27%) | 3 (5%) |
| 60. Cranial MRI starting at 7 days | 28 (50%) | 20 (36%) | 8 (14%) |
| 61. Cranial MRI a 3–4 days including diffusion-weighted MRI and MR spectroscopy | 21 (37%) | 16 (29%) | 19 (34%) |
| 62. Structural head ultrasound | 41 (73%) | 13 (23%) | 2 (4%) |
| 63. Head Doppler ultrasound | 33 (59%) | 13 (23%) | 10 (18%) |
| 64. Regional cerebral oxygen saturation | 12 (21%) | 10 (18%) | 34 (61%) |
| 65. Communication skills to improve empathic interaction with the family | 29 (52%) | 18 (32%) | 9 (16%) |
| 66. Techniques to improve teamwork | 25 (46%) | 20 (37%) | 9 (17%) |
| (b) Educational interventions in your unit | Very necessary | Desirable but not needed | Not needed |
|---|---|---|---|
| You consider training in the following tools | |||
| 67. Interpretation of neurologic examination | 31 (54%) | 21 (37%) | 5 (9%) |
| 68. Amplitude-integrated electroencephalography | 31 (54%) | 20 (35%) | 6 (11%) |
| 69. Cranial MRI from 7 days | 29 (51%) | 23 (40%) | 5 (9%) |
| 70. Cranial MRI in the first 3–4 days including diffusion-weighted MRI and MR spectroscopy | 29 (51%) | 26 (45%) | 2 (4%) |
| 71. Structural head ultrasound | 18 (32%) | 27 (47%) | 12 (21%) |
| 72. Health Doppler ultrasound | 23 (40%) | 23 (40%) | 11 (20%) |
| 73. Regional cerebral oxygen saturation | 24 (42%) | 29 (51%) | 4 (7%) |
| 74. Communication skills to improve empathic interaction with the family | 31 (54%) | 19 (33%) | 7 (11%) |
| 75. Techniques to improve teamwork | 32 (56%) | 16 (28%) | 9 (16%) |
| To improve the care of NBs with HIE, you would consider the following | |||
| 76. Electronically submitting the results of tests or neurologic examination to an expert colleague | 20 (35%) | 27 (47%) | 10 (18%) |
| 77. Transferring the patient to a different facility | 2 (4%) | 7 (12%) | 48 (84%) |
| 78. Attendance to practical trainings on the holistic management of NBs with HIE | 37 (65%) | 16 (28%) | 4 (7%) |
| 79. Attendance to practical trainings on the neurologic examination | 34 (60%) | 20 (35%) | 3 (5%) |
| 80. Attendance to practical trainings on aEEG | 35 (61%) | 13 (23%) | 9 (16%) |
| 81. Attendance to practical trainings on structural and Doppler head ultrasound | 35 (61%) | 16 (28%) | 6 (11%) |
| 82. Attendance to practical trainings on MRI | 36 (63%) | 20 (35%) | 1 (2%) |
| 83. Holding multidisciplinary case conferences/reviews | 41 (73%) | 11 (20%) | 4 (7%) |
aEEG, amplitude-integrated electroencephalography; HIE, hypoxic-ischaemic encephalopathy; MRI, magnetic resonance imaging; NB, newborn; TH, therapeutic hypothermia.
Items regarding the information given to parents on the condition and prognosis of their children with HIE managed with therapeutic hypothermia, number of hospitals n (%).
| Nearly always | Often | Rarely or never | |
|---|---|---|---|
| Condition of the infant | |||
| 84. Information is given by the attending physician in charge of the patient | 56 (98%) | 1 (2%) | 0 |
| 85. Information is given by the resident physician caring for the patient | 11 (19%) | 14 (25%) | 32 (56%) |
| 86. Information is given by the incubator in the unit | 27 (47%) | 21 (37%) | 9 (16%) |
| 87. Information is given in a room adjacent to the unit | 14 (25%) | 28 (49%) | 15 (26%) |
| 88. Information is given in the presence of the nurse caring for the patient | 18 (31%) | 29 (51%) | 10 (18%) |
| 89. The nurse is acquainted with the severity of the patient's illness before information is given to parents | 40 (70%) | 16 (28%) | 1 (2%) |
| Prognosis of the patient | |||
| 90. Information is usually given between 24 and 72h | 12 (21%) | 22 (39%) | 23 (40%) |
| 91. Information is usually given more than 72h post birth | 26 (46%) | 20 (36%) | 10 (18%) |
| 92. Information is not given until a clinical assessment and MRI are made close to discharge | 3 (5%) | 10 (18%) | 44 (77%) |
| 93. Information is established individually by the physician in charge of the NB | 9 (16%) | 5 (9%) | 43 (75%) |
| 94. Information is established following a meeting with other colleagues in the unit | 36 (63%) | 16 (28%) | 5 (9%) |
MRI, magnetic resonance imaging; NB, newborn.
Items on the usefulness of the different tools for the prediction of death or severe disability employed in the management of NBs with HIE in Spain.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 95. Neurologic outcomes in the first 3 days | 0 | 4 | 6 | 13 | 33 |
| 96. Perinatal variables: cord blood pH, Apgar, resuscitation | 2 | 22 | 18 | 10 | 4 |
| 97. Information from aEEG in the first 3 days | 4 | 1 | 5 | 20 | 26 |
| 98. Regional cerebral oxygen saturation in the first 3 days | 27 | 8 | 9 | 8 | 3 |
| 99. Head structural ultrasound findings | 2 | 13 | 23 | 15 | 3 |
| 100. Head Doppler ultrasound findings | 4 | 6 | 17 | 21 | 8 |
| 101. Findings of cranial MRI performed within 3 days of birth | 8 | 9 | 15 | 17 | 7 |
| 102. Findings of cranial MRI performed at 7 days or later | 0 | 0 | 2 | 13 | 41 |
| 103. Neurologic biomarkers | 22 | 9 | 13 | 9 | 2 |
| 104. EEG tracings at the end of the first week of life | 1 | 7 | 7 | 25 | 16 |
| 105. Visual evoked potentials | 21 | 6 | 16 | 9 | 4 |
| 106. Examination at discharge | 1 | 1 | 3 | 19 | 32 |
Answer on a 1 to 5 scale, where 1 stands for “not at all useful” and 5 “maximum possible usefulness”. The numbers in each cell represent the number of hospitals which gave that score.
aEEG, amplitude-integrated electroencephalography; EEG, electroencephalography; HIE, hypoxic-ischaemic encephalopathy; NB, newborn.
Items on discharge from hospital and followup, number of hospitals n (%).
| Nearly always | Often | Rarely or never | |
|---|---|---|---|
| Concerning follow-up | |||
| 107. There is a specific protocol for follow-up in the unit | 39 (70%) | 9 (16%) | 8 (14%) |
| 108. Follow-up carried out in every case of moderate-severe HIE | 54 (96%) | 2 (4%) | 0 |
| 109. Follow-up carried out in every case of mild HIE | 27 (48%) | 20 (36%) | 9 (16%) |
| 110. Follow-up through age | |||
| 2 years | 16 (28%) | – | – |
| 3–6 years | 23 (40%) | – | – |
| >6 years | 18 (32%) | – | – |
| 111. Follow-up carried out by a paediatric neurologist | 41 (73%) | 6 (11%) | 9 (16%) |
| 112. If carried out by a neonatologist, the neonatologist is specifically trained in neurodevelopment | 23 (41%) | 18 (32%) | 15 (27%) |
| 113. Multidisciplinary support: rehabilitation specialist, speech therapist etc. | 40 (71%) | 9 (16%) | 7 (13%) |
| 114. Uses appointments as an opportunity to explore coping and environmental factors with the family | 16 (29%) | 9 (16%) | 31 (55%) |
| The discharge report | |||
| 115. Includes all the tests performed | 54 (96%) | 2 (4%) | 0 |
| 116. Describes the neurologic condition at discharge | 52 (93%) | 4 (7%) | 0 |
| 117. Includes comments on patient prognosis | 7 (13%) | 12 (21%) | 37 (66%) |
| 118. Includes recommendations for follow-up care based on the patient's condition at discharge | 38 (68%) | 13 (23%) | 5 (9%) |
| 119. Specifies a follow-up plan and the specialists that will take care of the patient after discharge | 53 (95%) | 3 (5%) | 0 |
HIE, hypoxic-ischaemic encephalopathy.
The study was approved by the research committee of the coordinating hospital.
ResultsAll 57 tertiary care hospitals with level II–III units that provided TH responded to the survey.
Available resources: use of hypothermia and holistic care of newborns with hypoxic-ischaemic encephalopathy (Tables 1 and 2)Active TH was provided with servo-controlled whole-body cooling systems in all hospitals except 1 that also used selective head cooling (Coolcap® system, Natus). Most hospitals used the Tecotherm® system (Tecotherm Neo, Inspiration Healthcare Ltd.) (70%) and Criticool® system (CritiCool System, MTRE, Yavne, Israel) (21%), and only 26% of hospitals had more than one cooling system.
More than 95% of hospitals had specific care protocols for the implementation of TH. Blood tests were done in 98% of hospitals to assess for complications associated with HIE or TH. Of all hospitals, 68% keep the patient nil per os and slightly more than 70% uses sedation nearly always, usually as a continuous infusion (86%) of fentanyl (96%) or morphine or a similar opioid (51%), although 43% of centres reported rarely using clinical scales to titrate the dose.
Neurological monitoring during TH was conducted by means of amplitude-integrated electroencephalography (aEEG) in 85% of hospitals, but 6 out of 57 hospitals did not have access to aEEG, and out of those that did, 76% only had one system. Only 16% of hospitals routinely monitored the regional cerebral oxygen saturation (rScO2).
When it came to the tools used to assess the extent of brain injury, in addition to the neurological examination, we found a universal use of the head ultrasound scan and magnetic resonance imaging (MRI) around the time of discharge. Few hospitals (29%) tested blood or cerebrospinal fluid (CSF) samples for the presence of markers of brain injury. All hospitals reported following up newborns with moderate-to-severe HIE after discharge, although not all had multidisciplinary teams in charge of this follow-up.
Use of tools for neurologic monitoring and assessment of brain injury (Table 3)Most hospitals had providers trained in neurologic examination. Although 95% of units reported having professionals on staff that had knowledge of the interpretation of aEEG, 20% reported frequent difficulty interpreting background patterns and 54% frequent difficulty recognising electrical seizures. Half of the hospitals reported that nurses rarely interpreted aEEG tracings.
Head ultrasound scans were performed during the acute phase of HIE in 81% of hospitals, frequently including Doppler ultrasound (91%). In addition, 63% hospitals performed serial daily conventional and Doppler ultrasound examinations, with exclusive performance by neonatologists in only 35%. Magnetic resonance imaging was performed routinely in every hospital, usually in house where the infant was hospitalised (91%).
Testing for detection of markers of brain injury was the assessment tool used least frequently, as it was performed only in 16 hospitals (29%). These tests were usually performed in CSF, generally in an external laboratory (73%), and results were rarely available within 48 hours of sample collection.
Training needs (Table 4)Hospitals reported that the knowledge of neonatologists was generally adequate for most assessment tools, but inadequate for rScO2 monitoring (61%) and the interpretation of early MRI findings (34%).
Hospitals considered very necessary for staff to receive practical training on neurologic examination (60%), aEEG (61%), ultrasound (61%) and MRI (63%). They also reported a need of training on the delivery of holistic care to NBs with HIE (65%) and of holding multidisciplinary case conferences/reviews (73%). It is worth highlighting that 82% reported that it would be very useful or necessary to submit the findings of neurologic examination electronically for consultation with an expert colleague.
Informing parents on the prognosis of newborns with hypoxic-ischaemic encephalopathy (Tables 5 and 6)Most hospitals (91%) reported that providers informed families about the prognosis after holding a meeting with other unit staff, although only 21% of hospitals offered such information between 24 and 72 hours post birth. Although nurses are usually acquainted with the severity of the patient's condition, only 31% of hospitals reported the nurse being present nearly always when this information was given to the parents.
Respondents considered the neurologic examination, aEEG monitoring and the cranial MRI after 7 days the most useful prognostic tools, and biochemical markers and the rScO2 less useful.
Post-discharge follow-up plan (Table 7)Nearly all hospitals followed up patients with moderate-to-severe HIE after discharge, and 48% also frequently followed up patients with mild HIE. The professional in charge of follow-up was usually a paediatric neurologist (84%), and only 41% of hospitals where a neonatologist was in charge of the follow-up reported that the neonatologist had specific training on neurodevelopment.
All hospitals included the findings of the tests performed and of the neurologic examination in the discharge summary, although 65% rarely added remarks on the patient's prognosis. They also reported often making recommendations on care, establishing a follow-up plan and determining which physicians will care for the patient after discharge.
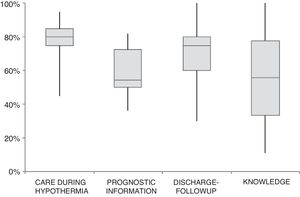
Fig. 1 summarises the quality of care to NBs with HIE in hospitals that use TH in Spain in terms of treatment variables, information about prognosis, discharge and post-discharge follow-up and staff knowledge.
Quality of the care delivered to newborns with HIE managed with therapeutic hypothermia in the 57 hospitals that offer therapeutic hypothermia in Spain. We assigned 1 point if the hospital answered “nearly always” to questions regarding management during hypothermia (items 1–9, 12–19, 22–23), prognosis (items 84, 88, 90, 95, 97–103, 106), post-discharge followup (items 107, 108, 111–119), and current knowledge (items 58–66). For items 95–106, with answers given on a scale of 1 to 5, we assigned a score of 1 if the answer given was 5. For each hospital, we calculated the total score in absolute terms by adding the scores of all the items, and also the total score as a percentage of the maximum possible score. Each boxplot shows the total scores calculated as a percentage of the maximum possible score for all hospitals for each of the dimensions under study.
The findings of our study suggest that the holistic care of NBs with moderate-to-severe HIE in tertiary hospitals that use TH in Spain is satisfactory overall, although there are areas for improvement.
Since the publication of national guidelines for the use of TH in Spain in 2011,9 this intervention has been introduced rapidly, so that it was used in 57 out of 90 existing tertiary care hospitals in 2015.8 However, there is currently no information on the overall management of HIE in Spain in relation to aspects as relevant as whether management follows a care protocol, the use of neurologic monitoring, the challenges that health providers experience when it comes to assessing the extent of injury or making a neurologic prognosis or whether tertiary care hospitals offer in-house follow-up care after discharge.3
When it came to material resources, we found that tertiary care hospitals in Spain that used TH had the necessary equipment to provide servo-controlled cooling and monitor the electrical brain activity of the NB during hypothermia. The advantages of servo-controlled systems in the delivery of TH are unquestionable in terms of effectiveness and safety.10 One relevant finding was that only 25% of hospitals had more than 1 system to deliver TH and only 26% more than 1 aEEG system. Although the number of systems that should be available per number of NBs in the caseload has not been established, a previous analysis found that in most of Spain, the number of TH and aEEG systems ranged from 1 per 6666 live births and 1 per 2857 live births, respectively.8 Considering that the incidence of HIE is of 1 per 1000 live births, the data suggest that the availability of equipment is insufficient at the population level, with the risk that it may not be possible to offer TH or that it will be provided without adequate monitoring if more than 1 patient need this intervention at the same time.
Neurologic monitoring with aEEG was the tool that supplemented TH most consistently in Spain. This reflects the value of aEEG along with the use of clinical scales for the identification of patients that need TH11–13 and the strong prognostic value of early aEEG.14 On the other hand, many of the electrical seizures experienced by these patients do not manifest as clinical seizures, and without the aEEG, it would not be possible to either detect them or assess their response to treatment.15 Unfortunately, 6 hospitals did not have aEEG available, and half of the hospitals that used aEEG monitoring did not use the information in real time, as nurses, the health care professionals that stay with the NB 24 hours a day, did not interpret the tracings or convey the resulting information to physicians. It is difficult to provide optimal care without the information provided by the aEEG, so it is essential to improve the training of nurses in the use of this monitoring technique.
In our study, the rScO2 and biomarkers of brain injury were the 2 tools used least frequently in clinical practice. There is evidence that some markers, such as protein S-100 or neuron-specific enolase in blood and especially in CSF, are very useful in patients with HIE.16–18 These markers are valuable from a practical standpoint because they help refine the prognosis at a very early stage when the usefulness of MRI is limited, and do not have the drawback of being affected by sedation. The aspect that needs to be improved in the use of these markers is the time it takes to obtain the results.
Neuroimaging tests are key to establish the location and extension of brain injury, have a strong prognostic value and are used all but universally in newborns with HIE in Spain. However, few hospitals used early structural and Doppler ultrasound scans around the time of TH initiation. At this time point, this imaging tests can be very helpful for ruling out structural abnormalities suggestive of metabolic disorders, infection or antenatal hypoxic-ischaemic injury.
When it came to MRI, its use was widespread in the first 6 weeks of life, when this technique can be used to obtain an individualised prognosis in patients with HIE.19,20 However, few hospitals (20%) reported the use of early MRI (first 4 days post birth) with diffusion-weighted imaging and magnetic resonance (MR) spectroscopy, when it allows a more precise estimate of the timing of the hypoxic-ischaemic insult and has a higher prognostic value.21–23
Establishing the prognosis of perinatal hypoxic-ischaemic injury requires that hospitals have these tools available and a staff with the necessary knowledge and experience to be able to interpret their findings. Prognosis in individual patients requires consistency in the information obtained using different methods, and informing the family of the prognosis is always challenging for health care providers.6,24 Therefore, it was no surprise that hospitals demanded additional education with specific practical courses on each of these diagnostic/prognostic tools.
Our findings evince that there are specific aspects of the management of these patients that could be improved.
Thirty percent of hospitals did not use sedation routinely, among other reasons for fear of causing respiratory depression, and half did not use clinical scales to monitor discomfort and pain. However, the stress and discomfort associated with TH can have a noxious effect and reduce the effectiveness of treatment.25–27 Although the clinical practice guidelines of the group on hypothermia (hipoSEN) of the Sociedad Española de Neonatología (Spanish Society of Neonatology), the group developed an addendum in which they recommended sedation of these patients.25 The clinical practice guideline on neonatal HIE of the Spanish Ministry of Health also recommends sedation, acknowledging that the basis of this recommendation is mainly the ethical imperative to alleviate the stress associated with cooling rather than the current scientific evidence.28
On the other hand, the practice of allowing parents to hold their children during TH is rare, despite evidence that this practice can be safe and also beneficial in that it promotes bonding.29,30
Seventy percent of hospitals did not introduce enteral feeding during TH, although evidence from some studies suggests that this is a safe practice31 that may reduce the length of stay and the time it takes to achieve exclusive oral feeding.32 However, it is important to take into account the scarcity of evidence on how to select patients eligible for enteral feeding and how to deliver it.3,33
In our study, we analysed when and how prognostic information is given to the parents. Only one third of hospitals conveyed this information in the first 72h of life, and nearly always in the absence of the nurse. These findings run counter to the need to receive early information30,34 and the important role of the nurse as the main source of information and communication support reported by parents in recent studies.35
Meeting the need for information and communication of parents of newborns with HIE is a complex challenge that requires a collaborative multidisciplinary approach. This complexity is perceived and voiced by health care professionals, as only half of the hospitals reported that their staff had adequate communication and teamwork skills and resources. Openly sharing information and maintaining effective communication are essential tools for the establishment of interdisciplinary cooperation. It is only when such a relationship is established that it is possible to deliver TH effectively and to develop rapport with the family in a way that can support them through the difficult experience that they are going through.
The timing of prognostication is key for the purposes of making decisions regarding withdrawal or withholding of life-sustaining treatment in some patients. If these decisions are delayed, it is possible that newborns with severe brain damage will survive with very severe disability that will limit independent function.36,37 Most patients with HIE die after treatment limitation,38,39 and therefore this decision must be made rigorously, responsibly and logically based on a thorough analysis of prognostic data and taking into account parental values.
We are happy to say that in Spain follow-up care was offered after discharge to all NBs with moderate-to-severe HIE. These infants require care from a multidisciplinary team, but it is helpful if a single member of this team is appointed to coordinate the follow-up and engage in more frequent and close interaction with the family.40 In our country, the main professional responsible for follow-up is the paediatric neurologist, but we found that when the professional in charge was a neonatologist, only one third of neonatologists had specific training on neurodevelopment. In addition, the duration of follow-up varied, and it is important that hospitals that only follow up patients through age 2 years increase this timeframe to 6 years of age, as cognitive and behavioural sequelae in these patients are usually detected between ages 2 and 6 years.40
There are several limitations to our study. The survey methodology carries a risk of bias, as it includes incomplete data in the analysis, and it is possible that some hospitals modified their care practices from the beginning of the study. One of the strengths of the study is that it contributes unique data that were previously unknown and from every hospital delivering TH in Spain, thus offering a comprehensive picture of the care offered newborns with perinatal HIE treated with TH in Spain.
In conclusion, our findings show that the care of NBs with perinatal HIE managed with TH is adequate overall and offers a general picture of the care delivered to these patients, but also revealed important opportunities for improvement. The availability of cooling and monitoring equipment needs to increase proportionally to the size of catchment populations, the training of professionals on the use of this equipment must improve, especially when it comes to the interpretation of aEEG by nurses, the use of sedation during treatment must be expanded, and the post-discharge follow-up of these patients must be extended through at least age 6 years in every hospital.
It is also important to offer education addressing the holistic approach to the management of NBs with HIE, including communication and interdisciplinary teamwork skills. Increasing competence in these areas could optimise physician-nurse collaboration, including the nurse in the delivery of information and in decision-making, and improve the management of complex issues, such as the timeframe in which parents are given prognostic information and allowing parents to hold their children during TH.
FundingThe authors did not receive any funding for this work.
Conflicts of interestThe authors have no conflicts of interest to declare.
This project was promoted by the NeNe Foundation, which was instituted as a not-for-profit organisation in 2015 and is devoted to the study and care of newborns with neurologic problems.
We thank Sara Calvo, the statistician at the Fundación Burgos por la Investigación de la Salud, for her contributions towards the improvement of the manuscript.
J. Diez-Delgado (Torrecárdenas, Almeria), I. Benavente-Fernández (Puerta del Mar, Cadiz), I. Tofé (Reina Sofía, Córdoba), A.E. Jerez (S. Cecilio, Granada), J.A. Hurtado (Virgen de las Nieves, Granada), J.M. Ceballos (J.R. Jiménez, Huelva), M.L. Millán (C. Hospitalario, Jaen), M.D. Esquivel (Materno-Infantil, Jerez), C. Ruiz (Costa del Sol, Marbella), M. Baca (Quirón, Malaga), E. Tapia (G. Universitario, Malaga), M. Losada (Quirón S. Corazón, Seville), E. Torres (Valme, Seville), A. Pavón (Virgen del Rocío, Seville), P.J. Jiménez (Virgen Macarena, Seville), F. Jiménez (S. Ángela de la Cruz, Seville), M.P. Ventura (Lozano Blesa, Zaragoza), S. Rite (Miguel Servet, Zaragoza), T. González (Cabueñes, Gijon), R.P. Arias (Central Asturias, Oviedo), P.R. Balliu (Son Espases, Majorca), J.M. Lloreda-García (S. Lucía, Cartagena), J.L. Alcaráz (Virgen de la Arrixaca, Murcia), C. Tapia (General, Alicante), A. de la Morena (S. Juan, Alicante), I. Centelles (General, Castellon), I. Güemes (Casa de Salud, Valencia), J. Estañ (Clínico, Valencia), A. Alberola (La Fe, Valencia), S. Aparici (Quirón, Valencia), R. López (Nisa 9 de Octubre, Valencia), J. Beceiro (Príncipe de Asturias, Alcalá de Henares), B. García (General, Fuenlabrada), L. Martínez (General, Getafe), E. González (Severo Ochoa, Leganés), L. Arruza (Clínico S. Carlos, Madrid), M.D. Blanco (Gregorio Marañón, Madrid), M.T. Moral (12 de Octubre, Madrid), B. Arias (La Zarzuela, Madrid), F. Mar (Ruber I., Madrid), J. Jiménez (Sanitas La Moraleja, Madrid), G. Romera (Montepríncipe, Madrid), A. Cuñarro (Alcorcón), C. Muñóz (Puerta de Hierro, Majadahonda, Madrid), F. Cabañas (H.U. Quirón, Madrid), E. Valverde (La Paz, Madrid), R. Montero (Nisa Pardo de Aravaca, Madrid), J.C. Tejedor (General, Móstoles), C. Santana (Materno Insular, Las Palmas), B. Reyes (Universitario de Canarias, Tenerife), S. Romero (N.S. de Candelaria, Tenerife), A. Orizaola (Marqués de Valdecilla, Santander), M. Baquero (General, Albacete), D. Hernández (General, Ciudad Real), A. Pantoja (Virgen de la Salud, Toledo), C. Vega-del-Val (HUBU, Burgos), L. Castañón (CAULE, Leon), E.P. Gutiérrez (Universitario, Salamanca), M. Benito (Clínico, Valladolid), S. Caserío (Río Hortega, Valladolid), G. Arca (Clínic, Barcelona), M.J. García (S. Creu i S. Pau, Barcelona), M.A. López-Vílchez (H. del Mar, Barcelona), L. Castells (General de Catalunya, Barcelona), M. Domingo (Parc Taulí, Sabadell), W. Coroleu (Germans Trias i Pujol, Badalona), H. Boix (Vall d’Hebron, Barcelona), R. Porta (I. Dexeus Quirón, Barcelona), A. García-Alix (S. Joan de Déu, Barcelona), S. Martínez-Nadal (SCIAS, Barcelona), E. Jiménez (Dr. J. Trueta, Girona), E. Sole (Arnau de Vilanova, Lleida), M. Albújar (Joan XXIII, Tarragona), E.M. Fernández (Infanta Cristina, Badajoz), A.R. Barrio (S. Pedro de Alcántara, Caceres), E. Piñán (Mérida), A. Avila-Alvarez (CHU A Coruña), M.E. Vázquez (Lucus Augusti, Lugo), N. Balado (CHU, Orense), P.A. Crespo (CHU, Pontevedra), M.L. Couce (Clínico, Santiago de Compostela), A. Concheiro-Guisán (Xeral, Vigo), I. Esteban (S. Pedro, Logroño), A. Lavilla (CH, Navarre), V. Alzina (C. Universidad de Navarra), A. Aguirre (Basurto, Bilbao), B. Loureiro (Cruces, Bilbao), I. Echániz (Quirón, Bilbao), M.D. Elorza (H.U. Donostia, San Sebastian), A. Euba (HUA Txagorritxu, Vitoria).