To assess performance of the age-adapted SOFA score in children admitted into Paediatric Intensive Care Units (PICUs) and whether the SOFA score can compete with the systemic inflammatory response syndrome (SIRS) in diagnosing sepsis, as recommended in the Sepsis-3 consensus definitions.
MethodsTwo-centre prospective observational study in 281 children admitted to the PICU. We calculated the SOFA, Pediatric Risk of Mortality (PRISM), and Pediatric Index of Mortality-2 (PIM2) scores and assessed for the presence of SIRS at admission. The primary outcome was 30-day mortality.
ResultsThe SOFA score was higher in nonsurvivors (P<.001) and mortality increased progressively across patient subgroups from lower to higher SOFA scores. The receiver operating characteristic (ROC) curve analysis revealed that the area under the curve (AUC) of the SOFA score for predicting 30-day mortality was 0.89, compared to AUCs of 0.84 and 0.79 for the PRISM and PIM2 scores, respectively. The AUC of the SOFA score for predicting a prolonged stay in the PICU was 0.67. The SOFA score was correlated to the PRISM score (rs = 0.59) and the PIM2 score (rs = 0.51). In children with infection, the AUC of the SOFA score for predicting mortality was 0.87 compared to an AUC of 0.60 using SIRS. The diagnosis of sepsis applying a SOFA cutoff of 3 points predicted mortality better than both the SIRS and the SOFA cutoff of 2 points recommended by the Sepsis-3 consensus.
ConclusionsThe SOFA score at admission is useful for predicting outcomes in the general PICU population and is more accurate than SIRS for definition of paediatric sepsis.
Analizar el rendimiento de la escala SOFA adaptada por edad en niños ingresados en la unidad de cuidados intensivos pediátricos (UCIP) y establecer si la escala SOFA puede competir con el síndrome de respuesta inflamatoria sistémica (SRIS) para el diagnóstico de sepsis, de acuerdo con las recomendaciones del consenso Sepsis-3.
MétodosEstudio prospectivo observacional en 2 centros en 281 niños ingresados en la UCIP. Se calcularon las puntuaciones de las escalas SOFA, Pediatric Risk of Mortality (PRISM) y Pediatric Index of Mortality-2 (PIM2) y se evaluó la presencia de SRIS al ingreso. La variable primaria fue la mortalidad a los 30 días.
Resultadosla puntuación SOFA fue más alta en los no supervivientes (p< 0,001) y la mortalidad incrementó progresivamente de los subgrupos con las puntuaciones SOFA más bajas a aquellos con las puntuaciones más altas. El análisis de las curvas de las características operativas del receptor (ROC) mostró que el área bajo la curva (AUC) para la predicción de la mortalidad a 30 días con la puntuación SOFA fue de 0,89, comparado con 0,84 y 0,79 en las escalas PRISM y PIM2, respectivamente. La AUC de la puntuación SOFA para la predicción de estancia prolongada en la UCIP fue de 0,67. La escala SOFA se correlacionó con las escalas PRISM (coeficiente de correlación de Spearman rs = 0,59) y PIM2 (rs = 0,51). En niños con infección, la AUC de la escala SOFA para la predicción de la mortalidad fue de 0,87, mientras que la AUC del SRIS fue de 0,60. El diagnóstico de sepsis definido como una puntuación SOFA de 3 o más predijo la mortalidad mejor que el SRIS y que la escala SOFA con el punto de corte de 2 puntos recomendada en el consenso Sepsis-3.
ConclusionesLa puntuación SOFA al ingreso es útil como predictor de la evolución en la población general de la UCIP y es más apropiada que el SRIS para definir la sepsis pediátrica.
In critically ill children admitted to the paediatric intensive care unit (PICU), the risk of morbidity and mortality is substantial in both developed and developing countries.1,2
Several prognostic scores have been developed with the aim of objectively quantifying the severity of disease and predicting the risk of death at the time of PICU admission, which can be very useful in treatment planning. Furthermore, such scores are essential to assess the quality of medical care, as much of the variation in mortality rates between PICUs is influenced by factors other than medical management, including the primary diagnosis and disease severity at admission.3,4
The ideal prognostic score should be accurate, simple, easy to use, minimally invasive and inexpensive.5 However, no paediatric mortality prediction score is completely satisfactory at present, and therefore researchers continue to devote significant effort to improving the accuracy of currently available scores and developing new ones.
The Pediatric Risk of Mortality (PRISM) and the Pediatric Index of Mortality (PIM), as well as their updated versions, are used widely to predict mortality.6,7 Other scores use organ dysfunction as a surrogate for mortality. Multiple organ dysfunction syndrome (MODS) is associated with a significantly higher risk of mortality,8 making it an excellent candidate marker of disease severity, with one study reporting a mortality rate of 1 % with dysfunction of 1 organ system with a progressive increase to up to 75 % in the presence of dysfunction of 4 organ systems.9
The pediatric organ dysfunction scores include the Pediatric logistic organ dysfunction score (PELOD)10, PELOD-2, and Pediatric Multiple Organ Dysfunction Score (P-MODS).11,12 The Sequential Organ Failure Assessment (SOFA) score (originally known as Sepsis-Related Organ Failure Assessment) is the score used most widely to quantify organ dysfunction in critically ill adults13 and has been recently considered to be intimately associated with the diagnosis of sepsis in adults (Third International Consensus Definitions for Sepsis and Septic Shock [Sepsis-3]).14 This has triggered an eager interest in researchers to adapt the SOFA to the paediatric population. Recently, a paediatric version of the SOFA score (pSOFA) was developed and validated retrospectively in critically ill children.15 However, there is limited data on the paediatric population and the pSOFA score has not yet been validated by means of prospective cohort studies or in low-income countries. The aim of our study was to assess the prognostic utility of the SOFA score in a heterogeneous cohort of critically ill children in a resource-limited country, and to determine whether the SOFA score can replace the long-held concept of Systemic Inflammatory Response Syndrome (SIRS) as the cornerstone of sepsis diagnosis in the paediatric age group.
Patients and methodsWe conducted a prospective observational study between March and November 2018 in the PICUs of 2 tertiary care hospitals in Egypt (Menoufia University Hospital and Atfal Misr Hospital). The study protocol was approved by the Committee for Medical Research Ethics of the Menoufia University School of Medicine, and we obtained informed consent from the parents.
We enrolled critically ill patients aged 1 month to 18 years by consecutive sampling. We excluded patients aged less than 1 month or more than 18 years. At the time of admission, the patient underwent a physical examination and a thorough history was taken. The laboratory tests performed included a complete blood count (CBC), blood gas analysis, measurement of levels of C-reactive protein (CRP), blood glucose and serum electrolytes, a coagulation profile and liver and kidney function tests. Cultures of body fluids, including blood, urine, cerebrospinal fluid (CSF) or pleural fluid, were performed based on the judgment of the clinician. Other laboratory and radiological investigations were performed where indicated based on the clinical condition of the patient.
In all patients, the paediatric SOFA score15 was calculated within 24h of admission. This score assesses 6 organ systems: respiratory, hematological, hepatic, cardiovascular, neurological and renal. A subscore of 0–4 points is calculated for each component, (Supplemental online content 1). We used the lowest value of each subscore in the first 24h to calculate total score. We gave a subscore a value of 0 if any of the data were missing. In addition to the SOFA, we calculated the PRISM6 and PIM-216 scores.
The number of SIRS criteria met by each patient was determined on admission, and we considered SIRS was present if a patient met 2 of the 4 criteria for SIRS, with 1 being either the change in the white blood cell count or in body temperature. The classic diagnosis of sepsis required the presence of SIRS in addition to suspected or proven infection. Severe sepsis was defined as sepsis with cardiovascular dysfunction, acute respiratory distress syndrome, or dysfunction in 2 other organ systems.17 We also applied the Sepsis-3 recommendations,18 according to which a diagnosis of sepsis requires an acute increase in the SOFA score of 2 or more points in association with proven or suspected infection. A patient was considered to have a suspected infection if antibiotherapy had been prescribed and cultures ordered. In the absence of documentation of previous organ dysfunction, we assigned a baseline SOFA score of zero.
We followed up patients until they were discharged from the PICU. The primary outcome was 30-day mortality. One secondary outcome was the composite variable 30-day mortality or PICU length of stay of 3 or more days. Another secondary outcome was the length of PICU stay in survivors.
Statistical methodsWe have expressed categorical data as absolute frequencies and percentages. We summarized continuous data as mean±standard deviation (SD) and compared them by means of the t test if they were normally distributed, and otherwise summarized them as median and range and compared them with the Mann-Whitney U test We used the chi square test to assess the association between categorical variables, and the Spearman correlation coefficient to assess the association between the SOFA score and different variables. We used univariate binary logistic regression was used to analyse the association of the SOFA score and other variables with outcomes, and multivariate logistic regression was used to adjust for confounding variables.
We used receiver operating characteristic (ROC) curve analysis to assess the power of the SOFA score to discriminate between survivors and non-survivors, calculating the Youden index to determine the optimal cut-off points for discrimination. The “area under the curve” (AUC) ranges between 0 and 1, and the closer the AUC of a variable is to 1, the better the performance of that variable. We assessed the calibration of SOFA score with the Hosmer-Lemeshow goodness-of-fit test to determine whether the predicted frequencies deviated from the observed frequencies of survivors and non-survivors. A p-value of 0.05 or greater in the goodness-of-fit test indicates that the model is well calibrated.
All statistical tests were two-tailed and we considered p-values of less than 0.05 statistically significant. In this document, we give the exact p-value unless it was extremely low, in which case we have expressed it as P < .0001. We performed the statistical analyses with the IBM Statistical Package for the Social Sciences, version 23 (SPSS Inc; Chicago, IL, USA).
ResultsPatient characteristicsWe enrolled 281 critically ill children in the study. Table 1 shows their basic characteristics. The 30-day mortality rate was 28.1 %. The standardized mortality ratio (SMR) was 3.09 (SMR=observed/expected mortality; expected mortality=median PRISM).
Demographic, clinical and laboratory data of the patients under study.
| Survivors(n=202) | Nonsurvivors (n=79) | Total sample(n=281) | P | |
|---|---|---|---|---|
| Age, months | 27.5 (1.3–216) | 11 (1.5–180) | 11 (1–216) | <.0001a |
| Male sex | 100 (49.5 %) | 48 (60.8 %) | 148 (52.7 %) | .089 |
| Weight, kg | 11 (2–83) | 6.5 (2.5–57) | 9.5 (2–83) | <.0001a |
| Primary reason for PICU admission Primary reason for PICU admission | ||||
| Gastrointestinal | 22 (10.9 %) | 3 (3.8 %) | 25 (8.9 %) | |
| Respiratory | 40 (19.8 %) | 24 (30.4 %) | 64 (22.8 %) | |
| Cardiac | 16 (7.9 %) | 15 (19 %) | 31 (11 %) | |
| Neurologic | 24 (11.9 %) | 9 (11.4 %) | 33 (11.7 %) | |
| Haematological/Oncological | 15 (7.4 %) | 4 (5.1 %) | 19 (6.8 %) | |
| Infectionb | 9 (4.5 %) | 10 (12.7 %) | 19 (6.8 %) | |
| Metabolic (DKA) | 36 (17.8 %) | 0 (0 %) | 36 (12.8 %) | <.0001a |
| Postsurgical recovery | 20 (9.9 %) | 4 (5.1 %) | 24 (8.5 %) | |
| Trauma | 7 (3.5 %) | 1 (1.3 %) | 8 (2.8 %) | |
| Otherc | 13 (6.4 %) | 9 (11.4 %) | 22 (7.8 %) | |
| Suspected infections | 93 (46 %) | 57 (72.2 %) | 150 (53.4 %) | |
| Positive culture | 27 (29 %) | 22 (38.6 %) | 49 (32.7 %) | .48 |
| Negative culture | 66 (71 %) | 35 (61.4 %) | 101 (67.3 %) | |
| Category according to SIRS | ||||
| Sepsis | 46 (22.8 %) | 50 (63.3 %) | 96 (34.2 %) | |
| Non-infectious SIRS | 77 (38.1 %) | 17 (21.5 %) | 94 (33.5 %) | <.0001a |
| No SIRS | 79 (39.1 %) | 12 (15.2 %) | 91 (32.4 %) | |
| MODS | 44 (21.8 %) | 64 (81 %) | 108 (38.4 %) | <.0001a |
| MV use | 47 (23.3 %) | 70 (88.6 %) | 117 (41.6 %) | <.0001a |
| SOFA score | 4 (0–12) | 10 (0–21) | 5 (0–21) | <.0001a |
| PRISM mortality risk% | 6.2 (0.6–98.9) | 44.1 (1.4–98.9) | 9.1 (0.6–98.9) | <.0001a |
| PIM2 mortality risk% | 1.6 (0.1–99) | 25 (0.1–99.5) | 2.3 (0.1–99.5 %) | <.0001a |
| WBC count, 1000/μL | 11.85 (1.9–31) | 13 (0.6–115) | 12.7 (0.6–115) | .93 |
| Haemoglobin, g/dL | 10.7±2.64 | 9.7±2.93 | 10.25±2.84 | .13 |
| Platelet count, 1000/μL | 300 (2–1047) | 181 (11–598) | 274 (2–1047) | <.0001a |
Data expressed as median (minimum-maximum), mean±standard deviation, or n (%).
DKA, diabetic ketoacidosis; MODS, multiorgan dysfunction syndrome (defined as simultaneous dysfunction of ≥ 2 organ systems); MV, invasive mechanical ventilation; PICU, paediatric intensive care unit; PIM2, Pediatric Index of Mortality score 2; PRISM, Pediatric Risk of Mortality score; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment Score; WBC, white blood cell; PICU, Pediatric Intensive Care Unit.
In the sample under study, 150 patients had suspected infection, which was confirmed by culture in only 34 %. Of the patients with infection, 96 had SIRS and were classified as cases of sepsis. Of the patients with sepsis, 40 were classified as having severe sepsis. Ninety-four patients had SIRS in the absence of infection (noninfectious SIRS). Ninety-one patients did not have SIRS (non-SIRS).
Admission to the PICU was most frequently due to respiratory disorders, especially pneumonia. Most patients admitted for postoperative recovery had undergone surgery for correction of congenital heart defects.
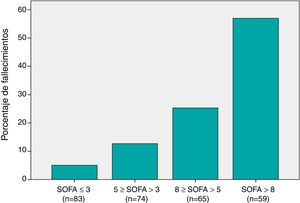
SOFA score and risk of 30-day mortalityThe SOFA score was significantly higher in non-survivors compared with survivors (Table 1). When we grouped patients based on the 25th, 50th and 75th percentiles of the SOFA score distribution (Fig. 1), we found that mortality increased progressively with the SOFA score (P < .001).
Distribution of 30-day mortality among the various SOFA subgroups: The x-axis shows the different patient subgroups based on the SOFA score. Patients were divided based on cut-off values corresponding to the 25th, 50th and 75th percentiles in the distribution of SOFA scores. The y-axis shows the percentage of patients that died in each subgroup relative to the total number of deaths.
First subgroup (SOFA≤3): 4 (4.8 %) of 83 patients died (5.1 % of the total number of deaths).
Second subgroup (5≥SOFA > 3): 10 (13.5 %) of 74 patients died (12.6 % of the total number of deaths).
Third subgroup (8≥SOFA > 5): 20 (30.8 %) of 65 patients died (25.3 % of the total number of deaths).
Fourth subgroup (SOFA>8): 45 (76.3 %) of 59 patients died (57 % of the total number of deaths).
The differences between the 4 subgroups were statistically significant (P <.001)
The univariate logistic regression analysis (Table 2) revealed that the SOFA score was positively associated with mortality, while weight and age exhibited a negative associations. In the multivariate analysis, the SOFA score was the only variable that remained independently associated with mortality.
Logistic regression analysis for prediction of 30-day mortality by SOFA score and other variables among the general PICU population.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | P | Adjusted OR | 95 % CI | P | |
| Age | 0.990 | 0.985–0.995 | < .0001a | 1.005 | 0.992–1.019 | .43 |
| Weight | 0.957 | 0.933–0.981 | < .0001a | 0.957 | 0.902–1.015 | .14 |
| SOFA | 1.61 | 1.43–1.80 | < .0001a | 1.59 | 1.42–1.79 | <.0001a |
CI, confidence interval; OR, odds ratio; SOFA, Sequential Organ Failure Assessment score.
The ROC curve analysis (Table 3), showed that the AUC of the SOFA score for discrimination between survivors and non-survivors was larger compared to the AUC of the PRISM and PIM2 and other laboratory markers.
Receiver operating characteristic curve analysis for prediction of 30-day mortality with the SOFA score and other predictors in the general PICU population.
| AUC | 95 % CI | P value | Cutoff level | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| SOFA | 0.886 | 0.840–0.931 | <.0001a | >6.5 points | 80.9 % | 81.8 % |
| PRISM | 0.840 | 0.780–0.900 | <.0001a | >17.2 % | 70.6 %% | 82.3 %% |
| PIM2 | 0.792 | 0.717–0.867 | <.0001a | >10.55 % | 60.3 % | 93.4 % |
| Platelet count | 0.646 | 0.568–.725 | .0004a | <266 500/μL | 64.7 % | 57.5 % |
| WBC count | 0.504 | 0.419–0.588 | .93 | >21 900 /μL | 19.1 %% | 89 % |
AUC, area under the receiver operating characteristic curve; CI, confidence interval; PIM2, Pediatric Index of Mortality score 2; PRISM, Pediatric Risk of Mortality score; SIRS, systemic inflammatory response syndrome; SOFA, Sequential Organ Failure Assessment Score; WBC, white blood cell.
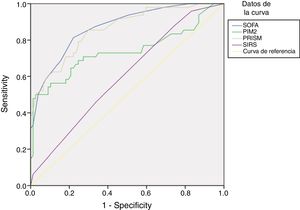
In the subset of patients with suspected infection, the SOFA score discriminated between non-survivors and survivors better than the PRISM and PIM2 scores and the presence of SIRS (Fig. 2).
Analysis of ROC curves for prediction of 30-day mortality using the SOFA score and other variables in patients with infection: In patients with infection, the AUC and 95 % CI for predicting mortality using the SOFA score were 0.871 (0.808 – 0.933), with P < .001, compared to an AUC of 0.602 using the SIRS (95 % CI, 0.502–0.702; P= .056), an AUC of 0.859 using the PRISM score (95 % CI, 0.792–0.925; P <.001) and an AUC of 0.738 using the PIM2 score (95 % CI, 0.637–0.839; P < .001). Applying a cutoff of 6.5 points, the SOFA score had a sensitivity of 81.3 % and a specificity of 77.9 % for discriminating nonsurvivors from survivors. Applying a cutoff of 2.5 met criteria, the SIRS has a sensitivity of 45.8 % and a specificity of 66.2 %.
The Hosmer-Lemeshow goodness-of-fit test yielded a p-value of 0.65, which indicates that the SOFA score is well calibrated. The p-values for the PRISM and PIM2 scores were 0.25 and 0.47, respectively.
Correlation between SOFA score and other variablesWe found that the SOFA score was positively correlated to the PRISM and PIM2 scores and the white blood cell count (WBC), and negatively correlated to age, weight, platelet count and hemoglobin concentration (Table 4).
Spearman correlation between the SOFA score and other variables in the patients under study.
| SOFA | ||
|---|---|---|
| Spearman correlation coefficient (rs) | P | |
| Weight | –0.318 | <.0001a |
| Age | –0.332 | <.0001a |
| PRISM | 0.59 | <.0001a |
| PIM2 | 0.51 | <.0001a |
| PICU stay | 0.214 | .002a |
| MV duration | –0.09 | .55 |
| WBC count | 0.16 | .014a |
| Platelet count | –0.31 | <.0001a |
| Haemoglobin | –0.24 | .0001a |
MV, mechanical ventilation; PICU, Pediatric Intensive Care Unit; PIM2, Pediatric Index of Mortality 2; PRISM, Pediatric Risk of Mortality; SOFA, Sequential Organ Failure Assessment Score; WBC, white blood cell.
The SOFA score was positively correlated to the length of stay in the PICU stay (Table 4). When we divided survivors into subgroups according to the median length of stay in the PICU into those with prolonged stays (>5 days) and those with short stays (≤ 5 days), we found that the SOFA score was significantly higher in the prolonged stay group (median, 5; range, 0–12) compared to the short stay group (median, 4; range, 0–10) (P < .001). The AUC for the SOFA score for predicting a PICU stay in survivors of more than 5 days was 0.67 (95 % CI=0.59–0.76; P <.001) compared to AUCs of only 0.48, 0.52, and 0.52 for the PRISM, PIM2 and SIRS, respectively.
We also divided the whole cohort according to the composite secondary outcome of death within 30 days or a length of PICU stay of 3 or more days. The SOFA score was significantly higher in the subgroup with the composite secondary outcome compared with the other subgroup (median and range, 5 [0–21] vs 3 [1–7]; P < .001). The AUC of the SOFA for prediction of the composite secondary outcome was 0.77 (95 % CI, 0.70 – 0.84; P < .001), compared to AUCs of only 0.45, 0.59, and 0.66 for the PRISM, PIM2, and SIRS, respectively.
SOFA, SIRS, and the definition of sepsisIn the patients with proven or suspected infection, the AUC of the SOFA score for predicting mortality was higher compared to the AUC of SIRS (Fig. 2).
Defining sepsis (applying the Sepsis-3 criteria) as the presence of infection plus a SOFA score of 2 points or greater, 93.3 % of patients with infection had sepsis, but the association of sepsis with mortality was not significant (Table 5). Applying this cutoff for the SOFA score, the mortality rate was 40 %, the sensitivity 100 % and the specificity 5.2 %.
Association of various sepsis definitions with 30-day mortality.
| OR (95 % CI) | P | |
|---|---|---|
| Sepsis defined as SOFA≥2 points | 6.00 (0.74–48.67) | .093 |
| Sepsis defined as SOFA≥3 points | 8.02 (1.80–35.67) | .006a |
| Sepsis defined as SOFA≥7 points | 13.73 (6.17–30.56) | <.0001a |
| Sepsis defined as SIRS | 4.46 (2.06–9.67) | .0002a |
CI, confidence interval; NA, not applicable; OR, odds ratio; SOFA, Sequential Organ Failure Assessment Score; SIRS, systemic inflammatory response syndrome.
Defining sepsis as a SOFA score cutoff of 3 points combined with infection, 84.7 % of patients with infection had sepsis, the sensitivity was 100 % and the specificity 18.2 %, and the association between sepsis and mortality became statistically significant (OR=8.02; P = .006).
Defining sepsis as a SOFA score of at least 7 points (the optimal cutoff for predicting mortality based on the ROC curve), 40 % of patients with infections had sepsis, and they were at higher risk of dying (OR=13.73; P <.001).
Defining sepsis based on meeting the criteria for SIRS (at least 2 criteria, with 1 being an abnormal temperature or WBC count) in the context of infection, the odds ratio for mortality was 4.46 (P <.001).
DiscussionThe development of an ideal paediatric prognostic score remains a challenging objective. In our study, we found that the SOFA score at admission was a good predictor of mortality in the overall PICU population. The median SOFA score was significantly higher in nonsurvivors compared to survivors and the mortality increased in a stepwise manner across patient subgroups from lower to higher SOFA scores. In addition, the association of the SOFA score with mortality continued to be significant in the multivariate analysis, while the association of age and weight with mortality did not. Moreover, the SOFA score had a good AUC of 0.886 for prediction of 30-day mortality, a finding consistent with a retrospective study in 8711 critically ill children in which the AUC of the SOFA score for predicting 28-day mortality was nearly identical at 0.88.15
Based on our data, the optimal SOFA cutoff for discriminating nonsurvivors from survivors was 7 points, compared to the cutoff of 8 points reported in a different paediatric study15 and the cutoff of more than 8 points described in a study in adults.19
Furthermore, we found that the SOFA score performed better compared to the PRISM and PIM2 scores in predicting mortality in the general PICU population, whereas another paediatric study reported an equal AUC for the SOFA score and a more recent version of the PRISM score (PRISM III).15
While the SOFA score performed well in predicting mortality, it was less successful in predicting the length of stay in the PICU in survivors and the combined outcome of mortality and length of stay in the PICU of 3 or more days. These findings are consistent with those of previous studies.20,21
Besides the superior performance observed our study, the SOFA score seems to offer additional advantages over the PRISM score. The SOFA score is available for free and does not require special software for its calculation. Furthermore, the SOFA score requires measurement of fewer parameters than the PRISM (6 vs 14). Also, the SOFA may be calculated daily, offering a dynamic assessment of disease progression, while the PRISM is only calculated at admission. The SOFA also does not require arterial blood gas measurements, which are difficult to obtain in children, as it was adapted to use SPO2 instead of PaO2 values.
On the other hand, one disadvantage of SOFA is that it is not calculated immediately on PICU admission and there is a turnaround time of 24h to get the worst value for each subscore. In this regard, the PIM2 score offers an advantage over the SOFA score, as the former is calculated within one hour of PICU admission, allowing earlier identification of high-risk patients that need more aggressive management. In addition, the SOFA score is relatively complex, which has led researchers to develop a quick version (qSOFA), but the latter seems to be less accurate.20,22 Unfortunately, it seems that improving one aspect of a score often comes at the expense of other aspects. There is hope that the discovery of more accurate prognostic biomarkers may improve or replace the currently available scores. Even with such developments, it will remain difficult to perfectly predict the disease course of each patient owing to the extreme complexity of the underlying immune, metabolic, and endocrine mechanisms, in addition to the effect of the genetic makeup on the individual’s response to treatment. Additionally, there is no guarantee that mildly ill patients will not deteriorate as a result of healthcare-related infections, adverse drug reactions or other care-related problems.
There has been a growing interest in the adaptation of the SOFA score to the paediatric age group following the recent change in the definition of sepsis in adults (Sepsis-3), by which the long-held construct of SIRS has been abandoned in favour of the SOFA score.14 In our study, the SOFA score proved to be more accurate in predicting mortality in patients with infection than SIRS (AUC, 0.87 vs 0.60). The SOFA score also proved superior to the PRISM and PIM2 scores. These findings are consistent with those of a recent multicentric retrospective study where the age-adapted SOFA score, calculated on admission, had an AUC of 0.83 in predicting mortality in children admitted to the PICU with proven or suspected infection, compared to an AUC of 0.73 for the SIRS.20 Similarly, another retrospective study reported that the SOFA score was more accurate than SIRS in predicting mortality in children admitted to PICU for infection.21
When we compared the SOFA score and SIRS specifically, we found that the odds ratio for the diagnosis of sepsis applying a SOFA cutoff of 7 for predicting mortality was 13.73, whereas the classic SIRS-based sepsis diagnosis predicted a lower risk of mortality (OR, 4.46).
Our findings, therefore, suggest that SOFA is superior to SIRS in defining paediatric sepsis. In fact, the concept of SIRS has been recently challenged, as it was found to suffer from excessive nonspecificity. In one study, nearly half of adult patients admitted to the ward developed SIRS at least once, making it impractical to use the SIRS criteria for early identification of sepsis.23 Another study described that 92 % of children visiting the emergency department with a fever of more than 38.5°C met the SIRS criteria, but most of them were discharged without requiring intravenous therapy or readmission.24 On the other hand, researchers realized that SIRS should not be the sole parameter for sepsis, as the pathophysiology of sepsis involves aspects other than excessive inflammation, such as activation of the anti-inflammatory response and changes in non-immunological pathways, including hormonal, metabolic, autonomic, and coagulation pathways, all of which have prognostic significance.14
In any case, our study found that a SOFA cutoff of 2 points, proposed by the Sepsis-3 group for diagnosis of sepsis, was not significantly associated with mortality and also had a very low specificity, which suggests that it is not possible to directly extrapolate the Sepsis-3 recommendations to the paediatric population, although other paediatric studies15,20 have confirmed the usefulness of this SOFA cutoff. Differences in sample size, setting or study design may explain this discrepancy, but it is important to keep in mind that the SOFA threshold of 2 points adopted in the Sepsis-3 criteria was selected a priori and not derived from ROC curve analysis. Furthermore, an important aspect has been criticised about the Sepsis-3 definition, which is that a patient is only considered to have sepsis when infection has reached a high degree of severity associated with organ dysfunction, which has a negative impact on the outcome.25 In other words, the new sepsis definition has improved specificity at the cost of sensitivity.26 This issue should be taken into consideration in establishing the SOFA cutoff to be used for diagnosis of paediatric sepsis, and suggests that a SOFA cutoff of 7 points should not be used to diagnose paediatric sepsis, even if this was the optimal cutoff for predicting mortality. Instead, based on the findings of our study, it seems more prudent to diagnose paediatric sepsis applying a SOFA cutoff of 3 points, which is associated with a greater likelihood of mortality compared to SIRS and at the same time allows physicians to diagnose sepsis relatively early, before organ dysfunction has progressed to higher levels of severity. We do not claim that the SOFA score has resolved the controversy surrounding the definition and diagnosis of paediatric sepsis. The SOFA score should be seen as a “step forward” rather than “a final solution” to the problem.
Overall, our findings suggest that the SOFA score should replace SIRS for the diagnosis of sepsis, although the applied cutoff in the SOFA should be slightly higher than the one proposed in the Sepsis-3 criteria for adults.
The limitations of our study include its small sample size, although this is counterbalanced by the very small p-values in our main results. We also used older versions of the PRISM and PIM2 scores. Furthermore, we did not calculate the SOFA score at different time points, when previous studies have reported that serial SOFA scores have predictive value.19 It makes sense for serial SOFA scores to predict mortality more accurately, as changes in the score would parallel the progressive increase in severity that precedes death. However, an earlier prediction of outcomes before the patient deteriorates to an irreversible state seems to be crucial. Last of all, the mortality in our cohort was high, presumably due to a lack of resources that frequently hindered the implementation of appropriate diagnostic and therapeutic interventions. Therefore, some of the findings of our study might to be generalizable to centres with low mortality rates.
ConclusionThe paediatric SOFA score was useful for predicting 30-day mortality in the general PICU population and performed better than the PRISM and PIM2 scores in this regard, but its performance was only fair in predicting a prolonged PICU stay. Additionally, the SOFA score should replace the criteria for SIRS for diagnosis of sepsis in the paediatric population, as it proved to be superior in predicting 30-day mortality in patients with infection. However, contrary to what is established in the Sepsis-3 definition, the optimal SOFA score cutoff for diagnosis of paediatric was 3 points rather than 2 points. Prospective multicenter studies are required for a better assessment of the paediatric SOFA score.
Please cite this article as: Mohamed El-Mashad G, Said El-Mekkawy M, and Helmy Zayan M. La escala pediátrica de evaluación del fallo multiorgánico secuencial (pSOFA): una nueva escala de predicción de la mortalidad en la unidad de cuidados intensivos pediátricos. An Pediatr (Barc). 2020;92:277–85.