Infantile haemangiomas (IH) are the most common benign soft tissue tumour of infancy. They affect 1–2.6% of newborns and up to 10% by 1 year of age.1 They typically present as solitary skin lesions. In some cases, IH may be multiple and involve extracutaneous organs, most commonly the liver.1,2
Hepatic haemangioma (HH) is the most frequent benign liver tumour in infancy,3 with a preponderance of female infants.4 They have been classified into three categories: focal, multifocal and diffuse.5 Focal HHs usually consist of solitary and asymptomatic lesions and generally do not require intervention.1 They seem to be equivalent to the skin lesions of rapidly involuting congenital haemangioma (RICH), and as happens in the latter condition, patients do not express the GLUT-1 glucose transporter that is usually found in IHs.1,5 Multifocal and diffuse HHs are true HHs. Multifocal HH presents as multiple spherical lesions that may remain asymptomatic until their spontaneous resolution, and less frequently they may lead to heart failure secondary to arteriovenous or portovenous shunting. In diffuse HHs, there is near-total replacement of liver parenchyma, which may trigger abdominal compartment syndrome due to mass effect. It may also be associated to hypothyroidism due to high production of type III thyronine deodinase.5
Traditionally, corticosteroids have been used as the first-line treatment of symptomatic HHs, alone or in combination with vincristine (VCR), which started to be used as an alternative to interferon, due to the latter's side effects,2 and cyclophosphamide.4 Embolisation and surgery are other possible treatment options.1
Since 2008, propranolol has been used successfully for the treatment of cutaneous IHs. It acts by stopping the growth of the lesion during the proliferative phase and inducing a rapid involution, effects that have not been observed with other therapies.1 Its efficacy and safety in the treatment of IHs has been demonstrated by an international randomised controlled trial that established a treatment dose of 3mg/kg/day.6
Table 1 shows the response to treatment with propranolol of different cases of multifocal and diffuse HHs published in the literature. Administration of propranolol was associated with bradycardia in one case,3 and no other adverse effects have been reported. When the dose was lowered, an increase in the size of the mass was observed despite continued corticosteroid therapy.3
Response to treatment with propranolol of cases of hepatic haemangioma published in the literature.
| Ref. | Case | Type of HH | Initial treatment | Response to treatment with propranolol |
|---|---|---|---|---|
| 1 | Girl, 6 weeks | Multifocal | – | Gradual and complete regression of one of the lesions at 5 and 13 weeks |
| Girl, 8 weeks | Multifocal | – | Partial regression at 2 months and complete regression at 16 monthsa | |
| Boy, 3 months | Diffuse | – | Significant regression by 4 months | |
| 2 | Boy, 3 weeks | Multifocal | – | Complete regression by 4 months |
| 3 | Girl, 15 weeks | Multifocal | – | Near-complete regression at 25 weeksa |
| 4 | Girl, 10 days | Multifocal | – | Complete regression at 1 month |
| 5 | Boy, 5 weeks | Diffuse | – | Partial regression at 20 daysa |
| Girl, 2 weeks | Diffuse | Corticosteroids | Near-complete regression at 3 years of age |
HH, hepatic haemangioma; Ref., bibliographic reference.
We present two cases of neonatal HH diagnosed and treated with propranolol in our hospital.
The first one corresponded to a full-term female newborn, large for gestational age and born to a diabetic mother (BDM). The abdominal ultrasound performed during the routine screening of newborns BDM revealed a liver lesion, with features compatible with HH in abdominal computed axial tomography (CAT) (Fig. 1A–C). Treatment with propranolol was initiated at 13 days post birth at a dose of 1mg/kg/day, which was increased gradually to 3mg/kg/day with no associated adverse effects. At 4 months of life, there were no signs of the liver lesion on ultrasound examination, and the beta blocker was tapered off. However, at 15 months a recurrence of HH with a 17mm lesion was observed adjacent to the vena cava, which resolved spontaneously. Currently, at 24 months of age, the tumour is in full remission.
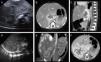
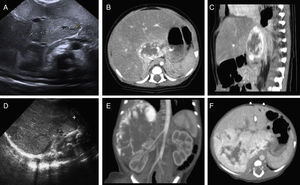
Case 1: (A) abdominal ultrasound: area of altered echogenicity in liver 37mm×21mm of axial diameter with increased vascularisation. CT angiogram: (B) axial plane, arterial phase. (C) Sagittal plane, portal venous phase. Lesion in hepatic parenchyma anterior to the vena cava in segment I and part of segments VII, IV and II, 43mm×22mm×34mm, with peripheral nodular enhancement in the arterial phase and centripetal fill-in in the portal venous phase. Case 2: (D) abdominal ultrasound: right hepatic lobe replaced by a homogeneous 90mm×50mm mass with multiple intralesional vessels, with additional focal alterations of echogenicity in the left hepatic lobe. CT angiogram: (E) coronal plane in the arterial phase. (F) Axial plane in the portal venous phase: large liver mass, 90mm×86mm×70mm, practically occupying the entire right hepatic lobe, sparing segments II, III and part of segment IV, showing marked peripheral nodular enhancement with centripetal progression during the portal venous phase. Several nodular foci with the same behaviour were also observed in the left hepatic lobe.
The second case corresponded to a full-term newborn girl with normal weight transferred to our hospital at 2 days of life for a mass in the right abdomen that extended to the right iliac fossa associated with significant abdominal distension and collateral circulation. Abdominal ultrasonography showed a mass in the right hepatic lobe, and the findings of abdominal CAT were compatible with diffuse HH (Fig. 1D–F). At 4 days of life, treatment with VCR, methylprednisolone and propranolol at 0.5mg/kg/day was initiated along with a pre-transplant evaluation. The ultrasound followup at 10 weeks of treatment showed a 70×50mm lesion, with barely discernible lesions in the left hepatic lobe, so VCR was discontinued and corticosteroid therapy was tapered off, continuing treatment with beta blockers alone from the second month of life at the maximum dose of 3mg/kg for a total duration of 15 months, which the patient tolerated well, with gradual reduction of the HH. Currently, at age 5 years, the patient is in full remission.
In conclusion, propranolol could be used as the first-line treatment of multifocal and diffuse HHs given its efficacy and safety, minimising the risks associated with other treatment options. We assume that, as occurs in cases of cutaneous IHs, it acts by stopping the proliferation of the mass and inducing its remission.
Please cite this article as: Baena-Gómez MA, Priego Ruiz MP, González EM, Rosa MJ, Muñoz Sánchez RM. Hemangiomas hepáticos: respuesta espectacular al tratamiento con propranolol. An Pediatr (Barc). 2015;83:435–437.