To assess glycaemic variability, oxidative stress and their relationship in children and adolescents with type 1 diabetes (T1DM) attending a summer camp.
Patients and methodsCross-sectional study that included 54 children and adolescents with T1DM aged 7–16, attending a 7-day summer camp. Sociodemographic information, clinical data, and blood glucose values measured using an Accu-Chek Nano® glucose metre were recorded. Glucose variability markers (standard deviation [SD], low blood glucose index [LBGI], high blood glucose index [HBGI], mean amplitude of glyacemic excursions [MAGE] and mean of daily differences [MODD]) were calculated. Oxidative stress was assessed by the measurement of 8-iso-prostaglandin F2 alpha (PGF2α) in a 24-h urine sample collected at the end of the camp in 14 children.
ResultsThe Median SD, MAGE and MODD indexes were in the high range (61, 131 and 58mg/dl, respectively), LBGI in the moderate range (3.3), and HBGI in the low range (4.5). The mean HbA1c was 7.6% and the median urinary excretion rate of 8-iso-PGF2α was 864.39pg/mg creatinine. The Spearman correlation coefficients between markers of glycaemic variability (SD, HBGI, MAGE, MODD) were significant. Non-significant correlations were found between markers of glycaemic variability and urinary 8-iso-PGF2α.
ConclusionsHigh glycaemic variability was observed in children and adolescents attending a summer camp. However, no correlations were found between markers of glycaemic variability and oxidative stress measured by urinary 8-iso-PGF2α. Further studies are needed to address the relationship between oxidative stress and glycaemic variability in children with T1DM.
Analizar variabilidad glucémica, el estrés oxidativo y la relación entre ambos en un grupo de en niños y adolescentes con diabetes tipo 1 (DM1) que asistieron a un campamento.
Pacientes y métodoEstudio transversal que incluyó a 54 niños con DM1 entre 7 y 16 años de edad que asistieron a un campamento de verano de 7 días. Se recogieron datos sociodemográficos, clínicos y valores de glucemia capilar medidos con un glucómetro Accu-Chek Nano®. Se calcularon los marcadores de variabilidad glucémica: desviación estándar (DE), índice de glucemia baja (LBGI), índice de glucemia elevada (HBGI), amplitud media de las excursiones glucémicas (MAGE) y media de las diferencias diarias (MOOD). El estrés oxidativo fue evaluado mediante la medición de 8-iso-prostaglandina F2 alfa (PGF2α) en una muestra de orina de 24h recogida en 14 niños al final del campamento.
ResultadosLa mediana de DE, MAGE y MOOD se encontraron en un rango elevado (61, 131 y 59mg/dl, respectivamente), LBGI en la categoría de riesgo moderado (3,3) y HBGI en la categoría de riesgo bajo (4,5). La media de HbA1c fue del 7,6%. La media de la tasa de excreción urinaria de 8-iso-PGF2α fue 864,39pg/mg creatinina. No se encontraron correlaciones estadísticamente significativas entre marcadores de variabilidad glucémica y 8-iso PGF2α urinario.
ConclusionesSe ha objetivado una alta variabilidad glucémica en niños y adolescentes con DM1 asistentes a un campamento de verano. Sin embargo, no se han encontrado correlaciones entre marcadores de variabilidad glucémica y de estrés oxidativo medido por 8-iso-PGF2α urinario.
Since the publication of the Diabetes Control and Complications Trial, HbA1c has been considered the main parameter for assessment of metabolic control in both children and adults with type 1 diabetes (T1DM).1 However, recent studies with continuous glucose monitoring (CGM) systems have revealed wide fluctuations in glucose values in children with T1DM, even in those with excellent HbA1c levels.2,3 Interest in studying the impact of these glucose fluctuations has therefore increased.4–6 The concept of glycaemic variability encompasses both diurnal variability (fluctuations in glucose within a single day) and day-to-day variability (fluctuations in glucose between one day and the next). Diurnal variability can be estimated using the standard deviation (SD) around the mean of glucose values for one day, and through the mean amplitude of glycaemic excursions (MAGE).7 The mean of daily differences (MODD)8 is a day-to-day variability marker. Other parameters for evaluating the risk of reaching extreme glycaemic values are the low blood glucose index (LBGI), which is a measure of the risk of hypoglycaemia,9 and the high blood glucose index (HBGI), which is a measure of the risk of hyperglycaemia.10
Glycaemic variability has recently been associated with an increase in the production of free radicals and oxidative stress.11 Isoprostanes are considered good markers of oxidative stress, as they are stable products derived from the process of oxidation of arachidonic acid by oxygen free radicals and have been shown to increase with oxidative damage.12,13 Isoprostanes can be estimated by measuring the 24-h urinary excretion rate of 8-iso-prostaglandin F2 alpha (8-iso-PGF2α).
The objectives of this study are to assess glycaemic variability and oxidative stress by the urinary excretion rate of 8-iso-PGF2α in a group of children and adolescents with T1DM attending a summer camp, and to analyse the relationship between both.
Patients and methodsPatients and study designWe did a cross-sectional study that included 54 children and adolescents with T1DM aged between 7 and 16, attending a 7-day summer camp in Málaga in July 2009. The summer camp was organised by a local diabetes association.
All the children gave verbal consent to participate in the study and their parents signed an informed consent.
MeasurementsWe collected sociodemographic and clinical data, including information on the duration of diabetes, the diabetes treatment and the presence of chronic diabetes-related complications. Diabetic retinopathy was considered to be present if there was a history of having received laser coagulation; diabetic nephropathy was considered to be present in patients with positive albuminuria requiring treatment with angiotensin-converting enzyme inhibitors or angiotensin II receptor antagonists, and diabetic neuropathy was considered to be present if the sensitivity of the hands and/or feet was altered or reduced.
During the camp, 7 blood glucose measurements were taken per day (one measurement before each meal [breakfast, lunch and dinner], one measurement 2h after each meal [breakfast, lunch and dinner] and one measurement at 3:00a.m.) from all participants, using the Roche Diagnostics Accu-Chek Aviva Nano® glucose metre. The glycaemia data stored in the glucose metres were transferred to a computer using the Roche Diagnostics Accu-Chek Smart Pix® device. For each participant, the Roche Diagnostics Accu-Chek Smart Pix® Data Management System software automatically calculated the percentage of blood glucose values in hypoglycaemia (glycaemia <70mg/dl [3.9mmol/l]), normoglycaemia (glycaemia 70–180mg/dl [3.9–10.0mmol/l]) and hyperglycaemia (glycaemia ≥180mg/dl [10.0mmol/l]), mean glycaemia, SD, LBGI and HBGI.
We collected data on the daily dose of insulin, the number of episodes of hypoglycaemia (glycaemia <70mg/dl [3.9mmol/l] with or without symptoms of hypoglycaemia14), number of episodes of severe hypoglycaemia (hypoglycaemia requiring the assistance of other persons14), number of episodes of ketosis (ketonuria measured using Bayer Ketostix® urine strips in children being treated with multiple daily insulin injections [MDII] and ketonaemia determined by an Abbott MediSense Optium Xceed Meter® in children with continuous subcutaneous insulin infusion [CSII] systems) and number of episodes of ketoacidosis that occurred during the camp.
The weight and height of participants were measured on the first day of the camp using standardised methods. Body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in metres). Z-scores were calculated for weight and BMI using recently published Spanish growth charts.15
At the start of the camp HbA1c was measured in a capillary blood sample using a DCA 2000 Analyzer® (Bayer Corporation, Elkhart, USA).
The 24-h urine sample was taken at home by each participant after the end of the camp. Although all the participants were requested to take a urine sample, only 14 children submitted it. The participants were asked to keep the urine samples refrigerated at 4°C during the collection process and to take them to the hospital within 24h of finishing collecting them. At the hospital the samples were immediately stored at −80°C after adding 0.005% BHT, since storage at −20°C is insufficient to prevent the formation of 8-isoprostanes. Measurement of 8-iso-PGF2α was carried out using an enzyme immunoassay method (Cayman Chemical Company, Ann Arbor, USA). The intraassay and interassay coefficients of variation were 15.2 and 18.5 respectively. The urinary creatinine level was determined using the enzyme spectrophotometric method based on alkaline picrate.
Assessment of glycaemic variabilitySD, LBGI and HBGI were calculated automatically for each participant by the Accu-Chek Smart Pix Data Management System® program using the blood glucose values recorded during the camp with the Accu-Chek Aviva Nano® glucose metre. The formulas for calculating LBGI and HBGI are published.9,10 The risk categories are LBGI: minimal (LBGI≤1.1), low (1.1<LBGI≤2–5), moderate (2.5<LBGI≤5.0) and high (LBGI>5.0), and HBGI: low (HBGI≤4.5), moderate (4.5<HBGI≤9.0) and high (HBGI>9.0).
MAGE is the mean of the absolute differences between the peak and the nadir of the glucose values over 24h, where peaks are defined as glucose values that precede an increase and are then followed by a decrease that exceeds mean glycaemia by more than 1 SD.7 A MAGE value of over 100mg/dl was regarded as high variability.
The MODD index was estimated as the absolute mean of daily differences in glycaemia using paired blood glucose values on successive days.8 The mean±SD of the MODD was calculated for each patient using the blood glucose values on 5 consecutive days. A MODD value of over 36mg/dl was considered as high day-to-day variability.8
Statistical analysisContinuous variables are expressed as mean±standard deviation or median and interquartile range, and categorical variables as percentages. The normal distribution of continuous variables was determined using the Shapiro–Wilk test. The two-group comparisons for continuous variables were determined using Student's t-test, or the Mann–Whitney non-parametric test where necessary. We calculated Spearman correlation coefficients to assess the correlations between study variables. We set a 95% confidence level for the two-tailed hypothesis tests.
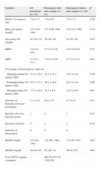
ResultsTable 1 shows the sociodemographic and clinical characteristics of the participants. As regards treatment, 3 of the children had CSII pumps with a rapid-acting insulin analogue, and the remaining children used MDII. Of the latter, 45 received a combination of long-acting insulin analogues (insulin glargine or detemir) and rapid-acting insulin analogues (insulin lispro, aspart or glulisine), and 6 received a combination of neutral protamine Hagedorn (NPH) insulin and rapid-acting insulin analogues (insulin lispro, aspart or glulisine). None of the participants exhibited microvascular complications.
Sociodemographic and clinical characteristics of the study population.
| Variables | Participants (n=54) |
| Age (years) | 11.41±2.33 |
| Sex | |
| Male (n [%]) | 25 (35.2) |
| Female (n [%]) | 29 (64.8) |
| Duration of diabetes (years) | 4.76±3.7 |
| Diabetes treatment | |
| MDII with NPH insulin (n [%]) | 6 (11.1) |
| MDII with long-acting insulin analogues (n [%]) | 45 (83.3) |
| CSII (n [%]) | 3 (5.6) |
| Weight (kg) (Z-score) | 44.6±11.8 (0.18±0.71) |
| BMI (kg/m2) (Z-score) | 19.23±2.66 (−0.69±0.66) |
| Daily insulin dose (IU/kg/day) | 0.85±0.26 |
Data are expressed as mean±standard deviation, unless otherwise specified.
BMI: body mass index; CSII: continuous subcutaneous insulin infusion; MDII: multiple daily insulin injections; NPH: neutral protamine Hagedorn.
A significant reduction in insulin requirement was observed during the camp (0.85±0.26IU/kg at the start of the camp vs 0.69±0.24IU/kg at the end, p<0.0001). Glycaemic control parameters, markers of glycaemic variability and episodes of acute diabetes-related complications are shown in Table 2. The mean number of episodes of mild hypoglycaemia per subject during the whole camp was 5.1 (range 0–17). One child had an episode of severe hypoglycaemia requiring intramuscular administration of glucagon to resolve it. Five episodes of ketosis were identified; these were treated at the camp and none of the cases developed into ketoacidosis. Of all children, 54% exhibited an HbA1c value ≤7.5% (58mmol/mol). The median SD, MAGE and MODD indexes were in the high range, mean LBGI was within the moderate risk category and mean HBGI was within the low risk category. 74% of the children had a MODD value of over 36mg/dl. LBGI values indicated a high risk of hypoglycaemia in 26% of the participants and a moderate risk in 39%. The median urinary excretion rate of 8-iso-PGF2α measured in 14 children at the end of the camp was 864.39pg/mg creatinine.
Markers of glycaemic control, glycaemic variability, episodes of acute complications and urinary excretion rate of 8-iso-prostaglandin F2 alpha in the whole study population and in groups, according to whether or not the 24-hour urine sample was submitted.
| Variables | All participants (n=54) | Participants with urine sample (n=14) | Participants without urine sample (n=40) | pa |
| HbA1c (%) (mean±SD) | 7.6±1.2 | 7.6±0.9 | 7.6±1.3 | 0.59 |
| Mean glycaemia (mg/dl) | 131 (110–146) | 121 (106–146) | 133 (113–146) | 0.38 |
| Glycaemia SD (mg/dl) | 61 (50–72) | 59 (40–74) | 61 (52–70) | 0.97 |
| HBGI | 4.5 (2.2–6.9) | 3.7 (1.2–7.4) | 4.8 (2.9–6.4) | 0.75 |
| LBGI | 3.3 (2.3–5.1) | 3.9 (3.3–6.0) | 2.7 (1.9–4.7) | 0.03 |
| Percentage of blood glucose values in | ||||
| Hyperglycaemia (%) (mean±SD) | 21.2±14.4 | 18.2±14.4 | 22.2±14.4 | 0.49 |
| Normoglycaemia (%) (mean±SD) | 61.5±17.2 | 59.2±16.8 | 62.3±14.4 | 0.98 |
| Hypoglycaemia (%) (mean±SD) | 17.3±10.5 | 22.7±8.8 | 15.5±10.5 | 0.01 |
| Episodes of hypoglycaemia per participant | 5.1±3.8 | 6.0±2.6 | 4.7±4.1 | 0.13 |
| Episodes of severe hypoglycaemia | 1 | 0 | 1 | 0.55 |
| Episodes of ketosis | 5 | 2 | 3 | 0.78 |
| Episodes of ketoacidosis | 0 | 0 | 0 | - |
| MAGE (mg/dl) | 131 (85–159) | 122 (68 –196) | 131 (94–153) | 0.92 |
| MODD (mg/dl) | 56 (41–67) | 63 (40–72) | 56 (41–67) | 0.64 |
| 8-iso-PGF2α (pg/mg creatinine) | – | 864.39 (723.39–1292.59) | – | – |
Data are expressed as median and interquartile range, unless otherwise specified.
SD: standard deviation; HbA1c: haemoglobin A1c; HBGI: high blood glucose index; LBGI: low blood glucose index, time in hypoglycaemia (glycaemia <70mg/dl), normoglycaemia (glycaemia between 70 and 180mg/dl) and hyperglycaemia (glycaemia ≥180mg/dl); MAGE: mean amplitude of glycaemic excursions; MODD: mean of daily differences; 8-iso-PGF2α: 8-iso-prostaglandin F2 alpha.
We found no statistically significant differences in markers of glycaemic variability (SD, MAGE, MODD and HBGI), HbA1c, duration of diabetes (4.79±3.28 years vs 4.75±3.18 years; p=0.98), age (11.86±1.91 years vs 11.25±2.46 years; p=0.33) and sex (50% male vs 42.5% male, p=0.63) between the children who submitted the 24-hour urine sample and those who did not (Table 2). LBGI and time in hypoglycaemia were significantly lower in the group of children who did not submit the urine sample (Table 2).
Table 3 shows the positive and statistically significant correlations that were observed between duration of diabetes, HbA1c, mean glycaemia, SD and the HBGI, and MAGE and MODD indices. LBGI correlated positively with MAGE and negatively with mean glycaemia. However, neither age nor urinary excretion rate of 8-iso-PGF2α correlated with markers of glycaemic variability (Table 3).
Spearman correlation coefficients between urinary excretion rate of 8-iso-prostaglandin F2 alpha, control markers and parameters of glycaemic variability.
| Age (years) | Time with diabetes (years) | HbA1c (%) | MG (mg/dl) | SD (mg/dl) | HBGI | LBGI | MAGE (mg/dl) | MODD (mg/dl) | 18-Iso-PGF2α (pg/mg creatinine) | |
| Age | 1 | |||||||||
| Duration of diabetes | 0.26 | 1 | ||||||||
| HbA1c | 0.21 | 0.48*** | 1 | |||||||
| MG | −0.15 | 0.47*** | 0.53*** | 1 | ||||||
| SD | −0.19 | 0.54*** | 0.52*** | 0.83*** | 1 | |||||
| HGBI | −0.17 | 0.55*** | 0.57*** | 0.95*** | 0.94*** | 1 | ||||
| LBGI | −0.86 | 0.24 | −0.47 | −0.34* | 0.11 | −0.11 | 1 | |||
| MAGE | −0.25 | 0.56*** | 0.38** | 0.61*** | 0.78*** | 0.73*** | 0.34* | 1 | ||
| MODD | −0.18 | 0.51*** | 0.58*** | 0.60*** | 0.74*** | 0.73*** | 0.15 | 0.58*** | 1 | |
| 8-iso-PGF2α | −0.34 | −0.27 | −0.12 | 0.13 | 0.01 | −0.002 | 0.12 | −0.24 | −0.26 | 1 |
SD: standard deviation; MG: mean glycaemia; HbA1c: haemoglobin A1c; HBGI: high blood glucose index; LBGI: low blood glucose index; MAGE: mean amplitude of glycaemic excursions; MODD: mean of daily differences; 8-iso-PGF2α: 8-iso-prostaglandin F2 alpha.
This study shows considerable glycaemic variability in children and adolescents with T1DM attending a summer camp. However, no correlations were found between high glycaemic variability and oxidative stress measured through the urinary excretion rate of 8-iso-PGF2α.
The objectives of the study included, on the one hand, assessment of glycaemic variability in children with T1DM, specifically in the context of summer camp. It is known that children and adolescents with T1DM are very prone to exhibit extreme glycaemic values, mainly due to variations in insulin sensitivity, level of physical activity and food intake.16,17 Specialised camps for children and adolescents with T1DM offer us an opportunity to study glycaemic excursions outside the hospital setting.18 For example, Choleau et al.19 assessed day-to-day glycaemic variability, estimated using the MODD index, which was calculated from blood glucose readings, in a group of 100 children with T1DM treated with MDII at a summer camp, reporting that the median MODD index was 77mg/dl and that in 99% of the participants the MODD value was over 36mg/dl. Another recent study20 of 48 children and adolescents with T1DM who underwent an ambulatory 3-day CGM showed high day-to-day glycaemic variability (MODD 63mg/dl in the group of children on MDII and MODD 61mg/dl in the group on CSII), and a high diurnal variability, especially in the group of children treated with MDII (MAGE 117mg/dl in the group of children on MDII and MAGE 88mg/dl in the group on CSII). Our results for glycaemic variability are consistent with data presented previously, and are even higher than those found in studies assessing children in their everyday lives, highlighting the fact that summer camps, even under the supervision of a specialist medical team, constitute an unusual situation.
On the other hand, we wanted to study oxidative stress and its relationship with glycaemic variability. Cerriello and Ihnat demonstrated that glycaemic variability can contribute to accelerated formation of free radicals,21 and furthermore Giacco et al.22 showed that oxidative stress can play an essential role in the development of diabetes-related microvascular and macrovascular complications, so this evidence indicates that glycaemic variability could have a greater deleterious effect on the development of complications than sustained hyperglycaemia.23 Nevertheless, the debate on the significance of glycaemic variability as a clinical finding in diabetes continues. Indeed, a recent study carried out on children with T1DM found no relationship between glycaemic variability assessed with CGM and vascular dysfunction.24 Monnier et al.25 demonstrated a close relationship between glycaemic variability and oxidative stress measured through urinary excretion rate of 8-iso-PGF2α in 21 subjects with type 2 diabetes (T2DM). However, it is difficult to replicate these results. DeVries's group, was not able to demonstrate this association in a group of adult patients with T1DM,26 nor in another group of patients with T2DM treated with oral antidiabetics (OAD).27 One possible explanation for the inconsistency of these results is that the methodology used to measure the urinary excretion rate of 8-iso-PGF2α differed between the two groups, as DeVries26,27 used tandem mass spectrometry, which is less prone to interference than the enzyme immunoassay technique used by Monnier.14 Another factor that could exert an influence is the potential effect of insulin on oxidative stress. In a recent study28 assessing subjects with T1DM and T2DM receiving various hypoglycaemic treatment regimens (OAD only, OAD with insulin and insulin only), it was found that patients being treated with insulin, alone or in combination with OAD, had a lower urinary excretion rate of 8-iso-PGF2α than patients treated with OAD only.
Our 8-iso-PGF2α urinary excretion results differ from those published by other authors, on which we have commented above, as they describe lower urinary 8-iso-PGF2α values in adult populations with T1DM or T2DM.25–28 However, there are fewer studies assessing oxidative stress and its relationship with T1DM in child populations. Gleisner et al.29 described an 8-iso-PGF2α urinary excretion rate of 1672±1706pg/mg creatinine, measured by enzyme immunoassay, in a group of children with T1DM of less than five years’ duration. However, they did not find statistically significant differences between the urinary excretion rate of 8-iso-PGF2α in these children with T1DM and 13 healthy controls paired for age and sex. In their study on 48 children and adolescents with T1DM, Schreiver et al.20 found a creatinine-normalized 8-iso-PGF2α urinary excretion rate of 2530±240pg/mg creatinine, measured by tandem mass spectrometry, and were unable to establish a correlation between glycaemic variability parameters and urinary excretion rate of 8-iso-PGF2α. The urinary 8-iso-PGF2α data we describe in our study are also lower than those found in these two studies on paediatric populations. In this case, although all the study subjects have T1DM and are being treated exclusively with insulin, the methodology used to measure urinary 8-iso-PGF2α is also different (enzyme immunoassay in our study and that of Gleisner vs tandem mass spectrometry in Schreiver's study), and other factors, such as the duration of the diabetes and the type of treatment (MDII vs CSII), are also different in this study and those referenced above, which could affect the results. In any case, further studies are needed, on the one hand to assess urinary 8-iso-PGF2α values in a healthy paediatric population in order to obtain reference values, and on the other, in homogeneous groups containing a larger number of children with T1DM.
The limitations of our study include its cross-sectional design and the high percentage of participants who did not take the urine sample, which means that the relationship between glycaemic variability and oxidative stress could not be properly assessed. The glycaemic variability parameters were estimated from the data for determinations of blood glucose and not from a CGM system; however, a recent study showed that the MODD index calculated from four blood glucose measurements in a group of children with T1DM correlated well (r=0.87) with the MODD index calculated from information collected by a CGM system, and concluded that blood glucose determinations could also be used to calculate the MODD index.19
In conclusion, high intraday and day-to-day glycaemic variability and moderately high urinary 8-iso-PGF2α excretion were observed in children and adolescents with T1DM attending a summer camp. However, we did not find correlations between markers of glycaemic variability and oxidative stress measured by the rate of urinary excretion of 8-iso-PGF2α. Further studies are needed to evaluate oxidative stress and its relationship with glycaemic variability in children with T1DM.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the Asociación de Diabéticos de Málaga (ADIMA: Málaga Diabetics’ Association), and especially José Sánchez, for their assistance.
Please cite this article as: Colomo N, Tapia MJ, Vallejo MR, García-Torres F, Rubio-Martín E, Caballero FF, et al. Variabilidad glucémica y estrés oxidativo en niños con diabetes tipo 1 asistentes a un campamento. An Pediatr (Barc). 2014;81:174–180.
Previous conference presentations: XXI Congreso de la Sociedad Española de Diabetes (Barcelona, 15–17 April 2010): Elevada variabilidad glucémica en niños con diabetes tipo 1 asistentes a un campamento de verano. 3rd International Conference on Advanced Technologies and Treatment for Diabetes (Basel, Switzerland, 10–13 February 2010). Standardised analysis of glycaemia and glycaemic variability in children with type 1 diabetes attending a summer camp. 34.° Congreso de la Sociedad Andaluza de Endocrinología y Nutrición (Granada, 5–7 November 2009). Análisis estandarizado de las glucemias y de la variabilidad glucémica en niños con diabetes tipo 1 asistentes a un campamento de verano.