Several publications highlight the usefulness of functional echocardiography (FnECHO) in neonatal intensive care. Data are lacking on its use in neonatal units in Spain.
ObjectivesTo evaluate frequency of use, patient characteristics, indications, measurements, and impact on patient management of FnECHO in a neonatal intensive care unit (NICU) in Spain over a 1-year period.
MethodsA retrospective study conducted in NICU patients during 1 year. Variables: gestational age, birthweight, admission criteria, days of life at examination, indication for FnECHO, parameters assessed, and treatment modifications.
Results168 echocardiographic studies were performed in 50 patients (mean 3.4; SD 2.83). The most frequent indication was patent ductus (PDA) assessment (58.3%), followed by haemodynamic instability (22.2%). The results of FnECHO modified treatment in 62 cases (36.9%). In 17.4% of them treatment with ibuprofen was initiated, and in 1.2% it was discontinued. In 10.8% of the cases, the results of FnECHO modified haemodynamic support. Echocardiographic evaluation included: assessment of presence/haemodynamic significance of PDA (100%); myocardiac function: ejection fraction/shortening fraction (EF/SF) 23.8%, left ventricular output 24.4%, right ventricular output 21.4%, systemic blood flow 42.3%; and signs of pulmonary hypertension 7.7%.
ConclusionsFnECHO is frequently used in the NICU, and in many cases it guides treatment. PDA assessment and haemodynamic instability are the most frequent indications. It still needs to be elucidated if the use of FnECHO modifies patient outcomes.
Numerosas publicaciones destacan la utilidad de la ecocardiografía funcional (EcoFn) en neonatología. No existen datos sobre su uso en unidades españolas.
ObjetivoEvaluar la frecuencia de uso, pacientes, indicaciones, mediciones y repercusión sobre el tratamiento de la EcoFn en un año en una unidad de cuidados intensivos neonatales (UCIN) española.
MétodosEstudio descriptivo retrospectivo en pacientes ingresados en UCIN en un año. Variables: edad gestacional, peso, diagnóstico principal, días de vida en el momento del estudio, indicación, parámetros medidos y modificaciones del tratamiento.
ResultadosSe realizaron 168 ecografías en 50 pacientes, con una media ± desviación estándar de 3,4 ± 2,83 por paciente. Las indicaciones más frecuentes fueron la valoración del ductus (58,3%) seguida de la inestabilidad hemodinámica (22,2%). El resultado de la ecografía modificó el tratamiento en 62 casos (36,9%). En un 17,4% se inició tratamiento con ibuprofeno y en un 1,2% de los casos se adelantó el fin de este. En un 10,8% de los casos, la ecografía modificó el soporte hemodinámico. Los parámetros principales valorados fueron: valoración de presencia/repercusión del ductus 100%; función miocárdica: fracción de eyección/acortamiento 23,8%, gasto ventrículo del izquierdo 24,4%, gasto del ventrículo derecho 21,4%; flujo sistémico 42,3%; signos de hipertensión pulmonar 7,7%.
ConclusionesLa EcoFn es utilizada frecuentemente en UCIN y en muchos casos guía el tratamiento de los pacientes. La valoración del ductus y de la inestabilidad hemodinámica son las indicaciones más frecuentes. Queda por determinar si el uso de la EcoFn modifica la evolución de los pacientes de UCIN.
The newborn may have a variety of haemodynamic problems with a variable and complex pathophysiology, that at times have limited clinical manifestations. This cardiovascular vulnerability stems from particular features of the newborn, such as incomplete myocardial development, the presence of foetal shunts, changes in systemic and pulmonary vascular resistance, and more generally the complex haemodynamic changes that take place during transition to extrauterine life.1 Despite progressive technological advances in neonatal intensive care, in most cases haemodynamic monitoring in newborns is still based on assessment of continuous heart rate, blood pressure (BP), acid–base status, urine output, or poorly validated clinical signs such as capillary refill time.2,3 While these measures provide important and useful information to the clinician, they are just variables associated in varying degrees to tissue perfusion, which is the key haemodynamic parameter, and for which an adequate monitoring method has yet to be developed.4 One potentially interesting option would be measurement of regional oxygen saturation by means of near-infrared spectroscopy, although further research is required to confirm this.5 Many publications have demonstrated that the correlation between BP and cardiac output is weak.3,6 It is not uncommon to find situations in which BP is normal and peripheral blood flow is below normal range. This can have important implications, as low peripheral blood flow in the first hours of life has been associated with the development of brain damage and neurodevelopmental abnormalities in premature infants.7,8 On the other hand, there is no clear definition of what constitutes a normal BP in newborns.9,10 The use of clinical assessment and the classic parameters mentioned before to elucidate the underlying pathophysiology in haemodynamic disturbances can lead to incorrect assumptions and therapeutic decisions, which may even cause harm.11
In the neonate, echocardiography is commonly performed by a cardiologist to assess structure and function of the heart. Usually, a single study is done to rule out congenital heart disease or global myocardial dysfunction, and the time at which it is performed often depends on the availability of a cardiologist. This can be an important factor in units that have limited access to a cardiologist, or in situations in which serial assessment is needed to evaluate response to treatment or the haemodynamic changes that take place during transition from foetal to postnatal circulation. It is important to consider that these changes can be very different from what might be assumed clinically based on conventional assessment.2 Functional echocardiography, or neonatologist-performed cardiac ultrasound, depending on the terminology suggested by different authors,2,12 is being introduced progressively in neonatal intensive care units (NICU) as a tool to help guide treatment choices. Functional echocardiography (FnECHO) adds to the assessment of classic clinical parameters in an attempt to provide individualised treatment based on the specific pathophysiology at hand. It must be used as an adjunct to clinical assessment and not as a substitute for assessment by a cardiologist, as their purposes are different and complementary. The neonatologist can use FnECHO to obtain information about haemodynamic function in real time and to make longitudinal assessments as needed.2 The aims of FnECHO are assessment of myocardial function, systemic and pulmonary blood flow, intracardiac and extracardiac shunts, and tissue perfusion.13 Functional echocardiography is commonly used to assess haemodynamic status during the transition from foetal to postnatal circulation in the extremely premature neonate, to assess for the presence and significance of PDA, to determine the underlying pathophysiology in patients with haemodynamic instability, and in neonates with high oxygen requirements.2,13–15 It has shown a positive impact on the management of adult patients.16–18 However, there is no clear evidence associating the use of FnECHO with improved outcomes of neonates admitted to the NICU, although some publications show a positive effect in this regard.11,19,20 As far as we know, there are no publications on the use of FnECHO in Spanish NICUs. We present the results of a retrospective study on the use of FnECHO in a Spanish NICU during a 1-year period, in which we analysed frequency of FnECHO use, type of patient receiving it, indications for FnECHO, and effects on the management of patients.
MethodsWe performed a retrospective study of every FnECHO done in newborns admitted to the NICU of the Hospital Clínico San Carlos of Madrid between January 2012 and January 2013. This 11-bed unit provides treatment for all types of neonatal disease except for heart surgery and ECMO (Level IIIB). All the studies were performed at the request of the physicians in charge by 2 NICU neonatologists (AC and LA) that have more than 5-year experience in FnECHO. The ultrasound assessments and measurements were performed according to a standardised procedure that has been previously described and is presented in Table 1.13,21 The indications for the ultrasound scans and their results were documented in the medical record of each patient. When an ultrasound was done, the results were discussed with the physicians in charge to decide on the therapeutic approach.
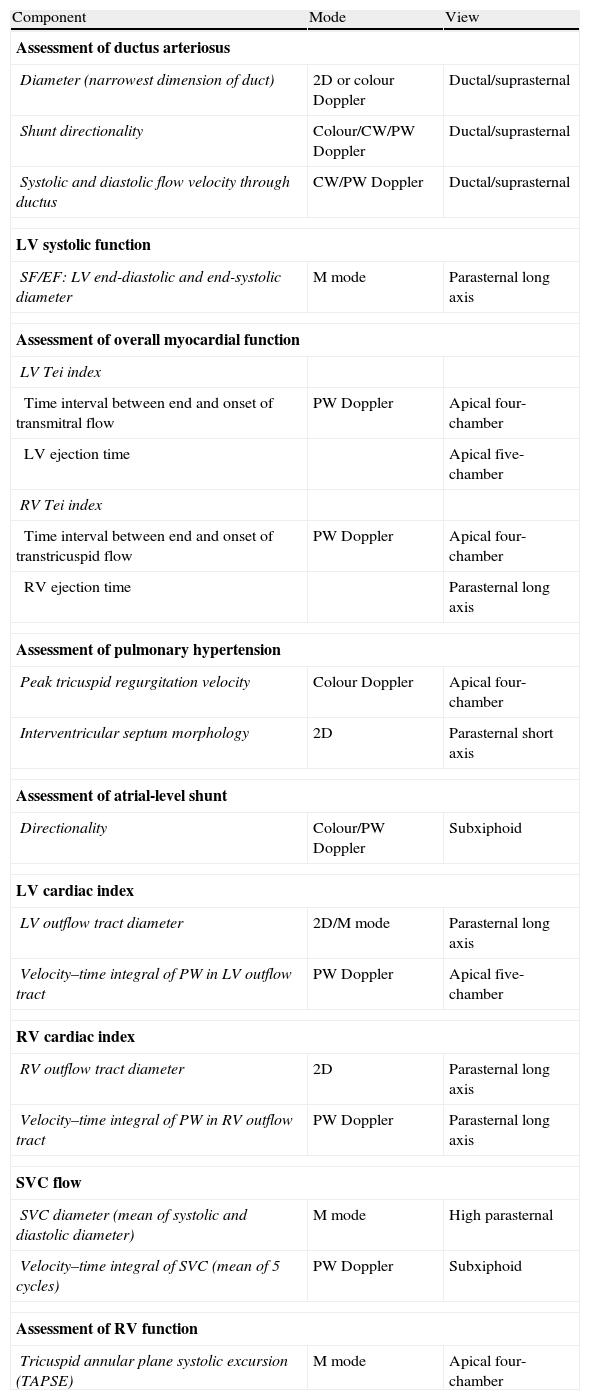
Components of functional echocardiography and techniques used for their assessment.
| Component | Mode | View |
| Assessment of ductus arteriosus | ||
| Diameter (narrowest dimension of duct) | 2D or colour Doppler | Ductal/suprasternal |
| Shunt directionality | Colour/CW/PW Doppler | Ductal/suprasternal |
| Systolic and diastolic flow velocity through ductus | CW/PW Doppler | Ductal/suprasternal |
| LV systolic function | ||
| SF/EF: LV end-diastolic and end-systolic diameter | M mode | Parasternal long axis |
| Assessment of overall myocardial function | ||
| LV Tei index | ||
| Time interval between end and onset of transmitral flow | PW Doppler | Apical four-chamber |
| LV ejection time | Apical five-chamber | |
| RV Tei index | ||
| Time interval between end and onset of transtricuspid flow | PW Doppler | Apical four-chamber |
| RV ejection time | Parasternal long axis | |
| Assessment of pulmonary hypertension | ||
| Peak tricuspid regurgitation velocity | Colour Doppler | Apical four-chamber |
| Interventricular septum morphology | 2D | Parasternal short axis |
| Assessment of atrial-level shunt | ||
| Directionality | Colour/PW Doppler | Subxiphoid |
| LV cardiac index | ||
| LV outflow tract diameter | 2D/M mode | Parasternal long axis |
| Velocity–time integral of PW in LV outflow tract | PW Doppler | Apical five-chamber |
| RV cardiac index | ||
| RV outflow tract diameter | 2D | Parasternal long axis |
| Velocity–time integral of PW in RV outflow tract | PW Doppler | Parasternal long axis |
| SVC flow | ||
| SVC diameter (mean of systolic and diastolic diameter) | M mode | High parasternal |
| Velocity–time integral of SVC (mean of 5 cycles) | PW Doppler | Subxiphoid |
| Assessment of RV function | ||
| Tricuspid annular plane systolic excursion (TAPSE) | M mode | Apical four-chamber |
CW: continuous wave; EF: ejection fraction; PW: pulsed wave; LV: left ventricle; MV: mitral valve; RV: right ventricle; SF: shortening fraction; SVC: superior vena cava.
We collected the following general data on patients: gestational age (GA), birth weight, and principal diagnosis. The following clinical data were recorded from the ultrasound tests: heart rate, presence of a murmur, presence of a gradient between preductal and postductal haemoglobin oxygen saturation >10%, mean BP (MBP; mmHg), lactate levels (mmol/L) and presence of oliguria (diuresis <1ml/kg/h).
The data about the echocardiographic examinations included: days of life at the time of examination, number of ultrasound studies per patient, and indication for examination. We documented whether the results of FnECHO led to treatment modification, and if so, what changes were made. Our analysis excluded ultrasound examinations performed in newborns with a suspected or confirmed structural congenital cardiopathy (CC). Although neonatologist-performed FnECHO, our unit is also used to localise catheters, assess for the presence of pleural effusion and its evacuation, the study of brain blood flow, or screening for gross brain injury, our study only took into account echocardiographic examinations.
Analysed ultrasound parameters: all echocardiograms were done with a portable ultrasound machine with a neonatal probe (Titan, SonoSite Inc., Bothell, USA, probe C11/5–8MHz): (1) assessment of ductus presence/consequences: presence, diameter in colour or 2D Doppler; shunt direction, ratio of left atrium to aorta, ductal Doppler pattern; (2) assessment of myocardial function and systemic blood flow: ejection fraction (EF), shortening fraction (SF), left ventricle (LV) and right ventricle (RV) cardiac output, superior vena cava blood flow and (3) assessment of pulmonary hypertension: calculation of pulmonary artery pressure in the presence of tricuspid regurgitation, interventricular septal shape, shunt direction.
Qualitative variables are expressed as absolute and relative frequencies, and quantitative variables as mean±standard deviation or median and interquartile range in case of asymmetrical distribution or high dispersion of the data. We used the statistical package STATA 12 to do the analysis.
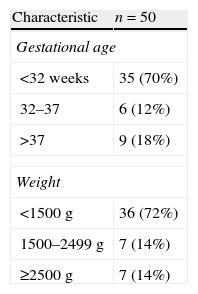
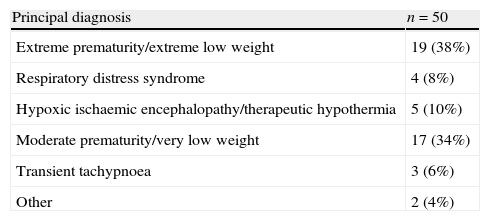
ResultsA total of 168 ultrasound examinations were performed in 50 patients during the period under study, with a mean of 3.4±2.83 examinations per patient. Table 2 shows the general characteristics of the patients. The mean GA of the patients was 31.3 weeks, with a range of 24.1–40.2 weeks, and the mean birth weight was 1549g (590–4160g). Seventy percent of patients were born before 32 weeks of gestation. The most frequent admission diagnosis was extreme prematurity and/or extremely low weight at birth (38%). Very low birth weight occurred in 34% of the patients (1000–1500g). Other principal diagnoses (Table 3) at the time of admission were hypoxic ischaemic encephalopathy to be treated with hypothermia (10%), respiratory distress syndrome (8%), transient tachypnoea (6%) and other (4%). The median days of life at the time of ultrasound examination was 6 days (2–19 days). The most frequent indication for echocardiography was assessment of the presence and significance of PDA, accounting for 58.3% of examinations (Table 4). Other indications included haemodynamic instability (22.2%), presence of heart murmur (8.3%), assessment of transition from foetal to postnatal circulation in extremely preterm neonates (5.4%), pulmonary hypertension screening (1.2%), and other (4.8%). As for the impact of FnECHO on patient management, the echocardiography results led to treatment modification in 62 patients (36.9%). The most common modifications involved the management of PDA. Thus, treatment with ibuprofen was initiated in 17.4% of patients following the ultrasound test, while in 1.2% of patients treatment with this drug was discontinued early. As for haemodynamic support, ultrasound results led to changes in the management of 10.8% of the patients. The changes involved volume expansion therapy (0.6%), initiation or modification of inotropic therapy (9.6%) or initiation of hydrocortisone therapy (0.6%). Out of all ultrasound scans, 36.3% were done in patients who had no murmur, arterial hypotension, oliguria, increased lactate levels, tachycardia, or presence of an oxygen saturation gradient. Of all these examinations, 21.7% corresponded to routine studies in the early days of life. Another 78.3% were follow-up studies in patients who had been previously assessed. In 14 of these ultrasounds (22.9%) the results led to a modification of treatment. Twelve corresponded to PDA. The analysis of echocardiography parameters included the assessment of the presence and significance of PDA in 100% of the patients. Myocardial function was assessed by means of the EF/FA ratio in 23.8%, LV output in 24.4% and RV output in 21.4% of patients. Systemic blood flow in the superior vena cava was measured in 42% of the examinations. The presence of pulmonary hypertension was assessed in 7.7% of patients.
Principal diagnoses of studied patients.
| Principal diagnosis | n=50 |
| Extreme prematurity/extreme low weight | 19 (38%) |
| Respiratory distress syndrome | 4 (8%) |
| Hypoxic ischaemic encephalopathy/therapeutic hypothermia | 5 (10%) |
| Moderate prematurity/very low weight | 17 (34%) |
| Transient tachypnoea | 3 (6%) |
| Other | 2 (4%) |
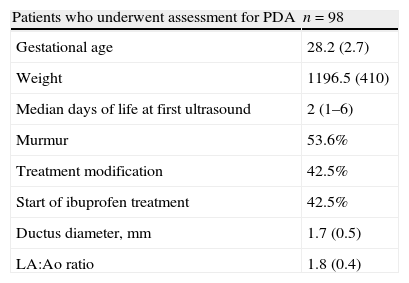
Characteristics of patients who underwent assessment for patent ductus arteriosus.
| Patients who underwent assessment for PDA | n=98 |
| Gestational age | 28.2 (2.7) |
| Weight | 1196.5 (410) |
| Median days of life at first ultrasound | 2 (1–6) |
| Murmur | 53.6% |
| Treatment modification | 42.5% |
| Start of ibuprofen treatment | 42.5% |
| Ductus diameter, mm | 1.7 (0.5) |
| LA:Ao ratio | 1.8 (0.4) |
Results are expressed as mean±standard deviation, percentage, or median with interquartile range.
Ao: aorta; LA: left atrium; PDA: patent ductus arteriosus.
This study is the first to describe the use of FnECHO in a Spanish NICU. The results suggest that this technique is used frequently and that it leads to treatment modification in a significant percentage of cases. A similar study published recently describes the performance of 512 FnECHOs in a Canadian unit over a period of 3.5 years, which works out at an average of 142 examinations a year, a figure that is very close to the one described in this work of 168 examinations in 12 months.22 That study also observed an increasing use of this technique during the period under analysis, which confirms the interest in FnECHO and its progressive implantation in neonatology units. A survey-based study shows that as early as 10 years ago 41% of the level 3 neonatology units in Australia and New Zealand had at least 1 neonatologist with FnECHO skills.23 This figure had increased by more than 90% in 2010.24 The neonatologist in our unit started to use FnECHO 10 years ago and used it mostly as a diagnostic tool and to monitor response to treatment of PDA. Since then, the range of indications has increased to the point that it has nearly turned into an additional part of the clinical assessment of haemodynamic disturbances. According to our study, the most frequent indication for FnECHO is diagnosis or monitoring of PDA (58.3% of patients), followed by assessment of pathophysiology underlying haemodynamic instability (22.2%). This is consistent with the data of the study by El-Kuffash et al., in which PDA assessment amounted for 51% of FnECHO indications.22
In our study, the results of 36.9% of FnECHO examinations led to changes in the management of the patient, a figure that is very close to the one reported by El-Kuffash, with a 41% rate of management changes following the ultrasound examination.22 Studies in adults suggest that FnECHO results in treatment changes in up to 51.4% of patients.25 This supports the definition of FnECHO as a tool that guides therapeutic decision making. In the study presented here, 36.3% of the ultrasounds were performed in patients with no other abnormal haemodynamic parameters or heart murmur, despite which the management of 22.9% of them changed after the examination. Almost all of these patients had PDA. We already mentioned that FnECHO findings are not always predictable based on traditional clinical assessment.2 Thus, El-Kuffash et al. found 6% of unexpected diagnosis.22 In fact, in FnECHO study performed in adult intensive care units, previously undetected severe abnormalities were found in up to 7.5% of patients.16 In our study, an average of 3.4 ultrasounds were made per patient, with some patients receiving up to 16 ultrasounds. This shows how FnECHO can be used for patient follow-up or to monitor response to treatment.2
While it is clear that this technique is useful in practise, strong evidence of the positive impact of the use of FnECHO in the NICU on patient outcomes is still lacking. But several retrospective studies11,19,20 and a few clinical trials26 suggest that FnECHO is beneficial in this way. In any case, randomised studies need to be performed to see whether the FnECHO has an effect on patient outcomes.
The main controversy surrounding performance of echocardiography by a neonatologist is based on the potential danger of missing a CC or making an incorrect diagnosis and starting the wrong treatment in a patient with CC. However, while this is a real danger, in most cases an existing structural cardiopathy is detected by FnECHO, even if a complete diagnostic assessment is not made.27,28 Either way, cardiologists and neonatologists must work in close collaboration, and a cardiology consult must be requested whenever there are doubts. Adequate training in FnECHO is essential to minimise these risks.2 Yet, despite the increasingly widespread use of this technique worldwide, many countries, including Spain, do not have a standardised training programme. In this case, training depends on personal interest, is performed at the same time as the working routine and relies on the collaboration of the cardiologists and/or the availability of specific materials like text books or CDs.29 This has been how training in FnECHO has been done in our unit, and it would not have been possible without the collaboration of paediatric cardiologists who understood that this technique is used by the neonatologist as an adjunct in clinical assessment, and not an intrusion in their field. Other countries like Australia, New Zealand, the United States, Canada, and some European countries have accredited training programmes.13,29,30 The American Society of Echocardiography, in collaboration with the European Association of Echocardiography and the Association for European Pediatric Cardiologists, has recently published specific recommendations for the training of neonatologists in FnECHO, which explain in detail what the training and accrediting process should entail,13 recommendations that have been modified by other authors.12 Given the growing interest in this technique and its widespread use, training and accreditation programmes should be developed in Spain with the support of the Sociedad Española de Neonatología (Spanish Society of Neonatologists).
There are several limitations to this study, most of which derive from its retrospective nature. For instance, treatment modification following FnECHO ultimately depended on the decision of the physician in charge of the patient, and not only on the data obtained from the ultrasound, which may have affected the perceived influence of this technique on the management of neonates, making it smaller or greater than it is. On the other hand, this study cannot determine whether therapeutic choices would have been different if FnECHO had not been available, nor whether those choices were the correct ones. Furthermore, the very decision of performing the ultrasound test, or not, also rested with the physician in charge rather than being determined by a study protocol, which could have a considerable effect on the observed frequency of use. Also, the echocardiographic view may constitute an important limitation in some patients.16 Although this study did not collect information on this variable in a systematic manner, technical limitations associated to the view and the use of a 5–8MHz probe rather than a probe with a higher frequency (8–12MHz), which would be ideal, may have affected the quality of some of these ultrasound studies.13
ConclusionsFunctional echocardiography is a useful tool employed widely in the NICU. It provides complementary information that aids in making therapeutic decisions. Its use is spreading in neonatology units, although clinical trials need to be done to assess its impact on patient outcomes. Structured training and accreditation programmes based on existing recommendations need to be developed to ensure the correct and safe use of this technique.
Conflicts of interestThe authors have no conflicts of interests to declare.
Please cite this article as: Corredera A, Rodríguez MJ, Arévalo P, Llorente B, Moro M, Arruza L. Ecocardiografía funcional en cuidados intensivos neonatales: experiencia en una unidad española a lo largo de un año. An Pediatr (Barc). 2014;81:167–173.