In developing countries, childhood cancer is the leading cause of death due to illness in the paediatric age group. Its incidence has been growing continuously since the 1950s1 due to advances in diagnostic tools and cancer registers, which has been associated with considerable improvements in prognosis and survival.
However, the cure of childhood cancer seems to have met a therapeutic ceiling, plateauing at 70% in the past few decades.2
We currently know that childhood cancer is a disease of multifactorial aetiology whose genetic basis is not entirely understood, with considerable involvement of the immune system and modulated by exposure to environmental factors.
At present, preventive measures are ineffective against childhood cancer. However, the detection of hereditary susceptibility could be very relevant to patients and families. In some cases, it could lead to the implementation of preventive measures for the early detection of malignancies, both in the index case and in blood relatives.
The genetics of cancerCancer results from the accumulation of different genetic changes in a cell, sometimes over several years. These changes lead to abnormal cell proliferation and clonal expansion, which can ultimately invade other tissues.
In most cases, genetic changes that promote tumorigenesis occur in somatic cells and do not involve germline mutations.
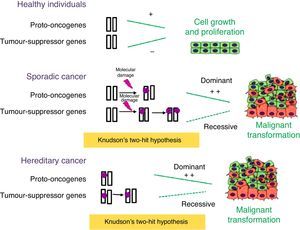
Numerous genes involved in tumour development have been identified, and are classified into 3 different categories: tumour-suppressor genes, proto-oncogenes and genes involved in genome stability. Tumour-suppressor genes control cell proliferation, inhibiting the progression of the cell cycle or inducing apoptosis (Fig. 1). Usually, a single functional copy of the gene suffices to carry out its function. The inactivation of both alleles allows uncontrolled proliferation and thus contributes to tumour development. Conversely, proto-oncogenes promote cell proliferation and contribute to tumour progression when they are permanently activated as a result of mutations. In this case, mutations in a single allele suffice to produce uncontrolled proliferation. Genes involved in DNA stability do not play a direct role in the regulation of cell proliferation, but dysfunction in these genes contributes to an increased number of mutations and thus to an increased probability of tumour development.3
Role of tumour-suppressor genes and proto-oncogenes in tumour development. Proto-oncogenes and tumour-suppressor genes function as antagonists: the former promote cell growth, while the latter inhibit cell proliferation. When sporadic or inherited mutations occur in proto-oncogenes, they become oncogenes that can orchestrate uncontrolled cell proliferation. According to Knudson's two-hit hypothesis, two mutation events need to take place in tumour-suppressor genes for tumour development to start. In hereditary cancer, the first mutation is inherited and thus the probability of having cancer is greater than that of the general population.
Broadly speaking, a hereditary susceptibility to cancer is suspected in families with 2 or more relatives in the same side of the family with the same type of cancer, several affected generations, earlier ages of diagnosis, individuals with multiple primary cancers, occurrence of different types of cancer that are genetically related (such as breast and ovarian cancer, or colon and uterine cancer), increased frequency of bilateral or multifocal as opposed to unilateral involvement, occurrence of nonmalignant and malignant changes in the same individual or family (for instance, Marfanoid habitus and multiple endocrine neoplasias type 2B). However, because of phenotypic variability and age differences in penetrance, many families may not fulfil these criteria.4
Most of the genes involved in the genetic predisposition to cancer are tumour-suppressor genes, and gain-of-function mutations in proto-oncogenes are only found in 10% of cases. In cases of predisposition due to mutations in tumour-suppressor genes, individuals inherit a defective copy of the gene, but based on Knudson's two-hit hypothesis, tumour development requires the somatic loss of the other allele. For this reason, mutations in tumour-suppressor genes are considered recessive. Since the probability of a mutation occurring in a single gene is higher than the probability of it occurring in the 2 alleles of the gene, the incidence of cancer in carriers of germline mutations of tumour-suppressor genes is much higher than that of the general population.
The case of carriers of dominant or gain-of-function mutations in proto-oncogenes is different. Although a mutation in a single allele suffices for cancer to occur, the presence of such a mutation does not necessarily result in the individual developing cancer. This will depend on the clinical penetrance of each type of tumour.
Genetic predisposition to cancer is due to mutations present in every cell of the body. These mutations, known as germline mutations because they were acquired via the germ cells, are prezygotic and may have been inherited or result from a de novo mutation in the germ cells of one of the progenitors (ova or spermatozoa). In the latter case, it would be the first time the mutation occurred in the family. In an individual without a genetic predisposition to cancer, mutations occur in somatic cells and are postzygotic.
Clinical implications of genetic predisposition to childhood cancerThe application of novel gene sequencing techniques in children with cancer is allowing us to expand our understanding of the molecular basis of childhood tumours. Unlike conventional molecular techniques, high-throughput techniques such as massive sequencing or next generation sequencing (NGS) can sequence millions of DNA fragments in parallel with progressively decreasing costs and shorter times. They can also detect different types of genomic changes with a single test.
A recent study included whole-genome sequencing by NGS of 1120 cancer patients aged 0 to 19 years. It found that 8.5% had germline mutations that predisposed to cancer, compared to 1% of the control group.5 The fact that there is a significantly higher percentage of germline mutations that predispose to cancer in the population of cancer patients raises several questions: would it be ethical and cost-effective to perform routine genetic screening of neonates with the purpose of identifying patients at risk of developing cancer? Would it be feasible to follow up all of these patients? Would they be able to benefit from an early diagnosis?
On the other hand, this same study found similar cancer histories in the families of patients with cancer and controls, which may be due to the young age of the parents, to the changes being de novo mutations (segregation analyses were not performed) or to incomplete documentation of the family history. This finding suggests that, on one hand, a positive family history should not be the sole indication for performing genetic testing in families, and on the other, that in some families, index cases may occur in the paediatric age group in the absence of adult cancers.
It would be interesting to conduct further studies to determine whether there is a high prevalence of inherited predisposing mutations. This would let us know whether detection of such mutations in probands would not only be useful to these individuals but also to their relatives. Genetic testing of relatives of cancer patients could identify other at-risk individuals that may benefit from early intervention, improving the associated morbidity and mortality.
We want to thank the Fundación Cris contra el Cáncer (http://www.criscancer.org/es) and the Fundación Uno Entre Cien Mil (http://unoentrecienmil.org/) for their support in this project.
Please cite this article as: Carrasco Salas P, Lapunzina P, Pérez-Martínez A. Predisposición genética al cáncer infantil. An Pediatr (Barc). 2017;87:125–127.