To determine the main clinical and epidemiological features of bacterial gastroenteritis in our environment.
Patients and methodsAn observational study of a Spanish population in 17 Autonomous Communities. Questionnaires of children with a stool positive culture to bacteria were collected over a one year period. A bivariate analysis was performed on the variables involved, as well as two multivariate models (for antibiotic treatment variables, and comparison Campylobacter/Salmonella).
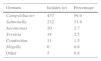
ResultsA total of 729 bacterial gastroenteritis episodes were recorded in the 17 Spanish autonomous regions, of which 41.2% were girls and 58.8% boys. The median age was 3.41 years old (interquartile range 1.55–6.72). The bacteria isolated were 59.9% Campylobacter, 31.8% non-Typhi Salmonella, 2.7% Aeromonas, 2.4% Yersinia, and 1.5% had more than one strain. Most infections (70%) were direct contacts, and food poisoning was less probable (25.9%). Salmonella is significantly less frequent than Campylobacter in children under the age of 3 years (adjusted OR 0.61; 95% CI: 0.43–0.86; P=.005), and Campylobacter is more frequent in rural areas (adjusted OR 1.48; 95% CI: 1.07–2.07; P=.012). Antibiotic was prescribed in 33.2% of cases. There was a greater significant difference if stools contained blood or mucus (adjusted OR 1.53; 95% CI: 1.04–2.27; P=.031), if the symptoms lasted more than 7days (adjusted OR 2.81; 95% CI: 2.01–3.93; P<.000), or if the child was admitted to hospital (adjusted OR 1.95; 95% CI: 1.08–3.52; P=.027).
ConclusionsThe aetiology of bacterial diarrhoea in paediatrics is typical of that of a developed country. The transmission mechanism is mainly direct, and more cases than appropriate are treated with antibiotics.
Conocer las principales características clínicas y epidemiológicas de la gastroenteritis bacteriana pediátrica en nuestro medio ambiente.
Pacientes y métodosEstudio observacional en el ámbito de la población española en 17 comunidades autónomas. Recogida de encuestas durante un año de niños con coprocultivo positivo a bacterias. Análisis bivariado y 2 modelos multivariantes (para las variables tratamiento antibiótico, y comparación Campylobacter/Salmonella).
ResultadosUn total de 729 episodios de gastroenteritis bacteriana en las 17 comunidades autónomas (41,2% mujeres y 58,8% varones). La mediana de la edad fue 3,41años (rango intercuartílico 1,55 a 6,72). El 59,9% de los aislamientos fueron Campylobacter, el 31,8% Salmonella no tifoidea, el 2,7% Aeromonas, el 2,5% Yersinia y más de un germen el 1,5%. La mayoría de contagios (70%) son directos, y la intoxicación alimentaria es más improbable (25,9%). Salmonella es significativamente menos frecuente que Campylobacter en menores de 3años (OR ajustada: 0,61; IC95%: 0,43 a 0,86; p=0,005), y Campylobacter es más habitual en el medio rural (OR ajustada 1,48; IC95%: 1,07 a 2,07; p=0,012). Se indicó antibiótico en el 33,2% de los casos, significativamente más si hubo productos patológicos en heces (OR ajustada: 1,53; IC95%: 1,04 a 2,27; p=0,031), duró más de 7 días (OR ajustada: 2,81; IC95%: 2,01 a 3,93; p<0,000), o se hospitalizó (OR ajustada: 1,95; IC95%: 1,08 a 3,52; p=0,027).
ConclusionesLa etiología de la diarrea bacteriana pediátrica es la propia de un país desarrollado. El mecanismo de contagio es principalmente directo, y se tratan con antibióticos más casos de los que parecería recomendable.
Although bacterial diarrhoea has decreased in importance as a paediatric infection in developed countries, it continues to be a significant cause of morbidity and hospitalisation.1 The most recent clinical practice guidelines (CPGs) and protocols mention a decline of bacterial aetiologies and a predominance of viral causative agents, and offer recommendations for its management,1–12 but few studies have analysed the actual approach to its management in primary care settings. According to data of the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) collected in 2013 for 28 members of the European Union and 4 non-members of the European Free Trade Association, Campylobacter was the bacterium isolated most frequently (214,779 reported cases during that year), followed by Salmonella (82,694) and, with a vast difference, by Yersinia (6,471) and verotoxigenic Escherichia coli (6,043).13 The figures are similar in children, although the incidence of acute gastroenteritis (AGE) is much higher in early childhood, with 0.5–2 episodes per child per year in children aged less than 3 years in the European Union.14
The latest report of the Sistema de Información Microbiológica del Centro Nacional de Epidemiología (Spanish Immunologic Surveillance System [SIM]), with data from 2013,15,16 showed that in children aged less than 15 years, of all bacterial isolates that could potentially cause gastroenteritis, 62.7% corresponded to Campylobacter, 34.8% to nontyphoid Salmonella and 2.4% to Yersinia. Thus, we have data on the aetiology and age distribution of bacterial AGE, but few updated data are available on its clinical manifestations and the approaches to its management.
In September 2013, the Asociación Española de Pediatría de Atención Primaria (Spanish Association of Primary Care Paediatrics) launched a Primary Care Paediatrics Epidemiological Surveillance Network (Red de Vigilancia Epidemiológica en Pediatría de Atención Primaria [PAPenRED]) with the purpose of conducting research on aspects related to child health in Spain. Through this network, we embarked on this study with the purpose of learning the current clinical and epidemiological characteristics of bacterial AGE in Spain, as well as the prevalence of antibiotic resistance in the most common causative agents.
Patients and methodsPAPenRED has a network of sentinel collaborators employed in public or publicly funded private primary care (PC) centres in every autonomous community (AC) in Spain, in numbers that are proportional to their respective populations. It comprehends 304 paediatricians that submit data through online questionnaires. For this study, we designed a questionnaire that can be found at http://bitly.com/GEA_PAPenRED, and collaborating paediatricians submitted data for each case of bacterial AGE diagnosed at their sites. We asked sentinels to simply report on these cases, without applying any specific criteria or recommending performance of tests beyond those already requested based on the clinical condition of the patient or the duration of symptoms. We accepted responses for the study between April 1, 2014 and March 31, 2015.
The inclusion criteria were: visiting the PC paediatrics clinic during the period under study, age 0–14 years, clinical manifestations of AGE, performance of stool culture at some point during the episode and availability of clinical and epidemiological data for the episode. The exclusion criteria were: lack of documentation in the health records or of access to the records, presence of intestinal or immune diseases that could predispose to diseases like AGE, and patients treated with immunosuppressive drugs.
The primary variables were the involved bacterium and having or not having received antibiotherapy. The secondary epidemiologic variables included age, sex, date of episode, school attendance, siblings, parental educational attainment, possible mechanism of transmission, setting (rural or urban) and presence of associated cases. The secondary variables pertaining to clinical manifestations and the course of disease were: presence of fever (axillary temperature >37.5°C), concurrent vomiting, stool characteristics, need for hospital admission and time elapsed to resolution of diarrhoea. We also analysed the findings of antimicrobial susceptibility testing.
Bacterial cultures and antimicrobial susceptibility tests for samples collected in the sentinel sites (168 PC centres) were performed in their respective reference microbiology laboratories.
In the statistical analysis, we described qualitative variables by calculating frequency distributions and percentages for each category. For quantitative variables, we checked the normality assumption using the Kolmogorov–Smirnov test and calculated measures of central tendency (mean or median) and dispersion (standard deviation or interquartile range).
In the bivariate analysis, we assessed the association between the different categorical variables and the primary outcomes, calculating odds ratios (ORs) with their respective 95% confidence intervals (CIs). We completed our analysis by fitting two logistic regression models; in the first one, the dependent variable was the presence of Salmonella or Campylobacter, while in the second one it was the use of antibiotherapy. We performed the analysis with the SPSS software, and defined statistical significance as a P-value of less than .05.
The PAPenRED project has been approved by the Committee on Ethics and Clinical Research of Aragón from its (Resolution in Favour, Minute n. 19/2013; CP-CI PI13/00154).
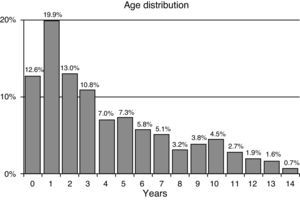
ResultsWe received 729 completed questionnaires (204 sentinels; 11 sentinels reported having had no cases during the study period). The cases corresponded to 299 girls (41.2%) and 430 boys (58.8%). Their ages ranged from 1 month to 14 years and 11 months, with a median of 3.41 years and interquartile range of 1.55–6.72 years (non-normal distribution, Kolmogorov–Smirnov test: Dmax=0.152; P<.000). The age of 56.3% was less than 4 years. Fig. 1 shows the distribution of episodes by age group.
In the 729 positive cultures (11 with coinfection), 59.9% of the isolates were Campylobacter species and 31.8%, nontyphoid Salmonella species (Table 1). Cases of bacterial coinfection included 7 cases of Campylobacter associated with Aeromonas, 2 of Campylobacter associated with Salmonella, 1 of Campylobacter with Yersinia and 1 of Salmonella with Aeromonas. Viral coinfection was found in 8 cases: rotavirus in 5, adenovirus in 2 and astrovirus in 1.
Only 14.8% of episodes took place in winter. Campylobacter was isolated more frequently in spring (35.9% of Campylobacter isolates), while Salmonella and Yersinia were proportionally more frequent in summer (37.5% and 38.9% of cultures, respectively) and Aeromonas in autumn (40% of isolates).
Of all patients, 72.6% attended school or a childcare centre. To assess the impact of schooling, we studied the distribution of microbes in children aged less than 4 years, of who no more than 30.3% were enrolled in a facility. The distribution was similar, although of the few patients infected by Yersinia (n=9), 88.9% attended a facility.
There was a higher probability of infection by Salmonella in children that had siblings compared to single children, although the difference was not statistically significant (35% vs 28%; OR, 1.35 [95% CI, 0.97–1.87; P=.093).
When it came to the different ACs, Catalonia, the Balearic Islands and the Canary Islands were underrepresented in the study (they contributed a number of cases of less than two thirds the proportion expected based on the corresponding population proportions). The proportion of Salmonella infections was higher than the proportion of Campylobacter infections in Asturias, Castilla-La Mancha and the Canary Islands (51.8%, 51.3% and 63.6% of salmonellosis cases, respectively). Children in these three ACs had ORs of having AGE due to Salmonella that were significantly above the national average: 2.26 in Asturias (95% CI, 1.04–4.88; P=.040); 2.21 in Castilla-La Mancha (95% CI, 1.14–4.29; P=.011) and 3.67 in the Canary Islands (95% CI, 1.06–12.65; P=.012). The proportion of Campylobacter cases in Galicia was significantly greater than the national average (OR, 2.65; 95% CI, 1.21–5.83; P=.021).
In rural settings, more patients had Campylobacter (55%) compared to Salmonella (47.3%) (OR, 1.36; 95% CI, 0.98–1.89). The prevalence of Aeromonas was significantly higher in rural settings compared to urban settings (78.9% vs 52.8%; OR, 3.36; 95% CI, 1.10–10.22; P=.040).
The suspected mechanism of transmission in cases in which a potential source was reported (66%) was contaminated food in a greater proportion of cases of Salmonella (33%) compared to Campylobacter (23%), and the difference was statistically significant (OR, 1.66; 95% CI, 1.07–2.57; P=.004). For the overall case series, transmission was believed to have occurred through direct contact in 70% of cases, and through contaminated food in 25.9% of cases.
Table 2 presents the setting where transmission took place for those cases in which it was known (n=326) and by pathogen. In cases of suspected foodborne illness, the most frequent sources of Campylobacter were poultry meat (28%) and eggs (28%) followed by other meats (19%), while the most frequent sources of Salmonella were eggs (45%) fish or meat (30%) or dairy products or baked goods (21%).
Probable setting of transmission based on the aetiological agent in the cases in which it was known (n=326), expressed as percentages.
| Setting of transmission | Campylobacter | Salmonella | Aeromonas | Yersinia | Coinfection |
|---|---|---|---|---|---|
| Patient's home | 37.7% | 43.0% | 14.3% | 11.11% | 60.0% |
| Another home | 14.1% | 11.4% | 28.6% | 0.0% | 0.0% |
| Child care/school | 32.5% | 24.6% | 42.9% | 66.7% | 40.0% |
| Bar/restaurant | 7.8% | 14.9% | 0.0% | 11.1% | 0.0% |
| Leisure centres | 7.8% | 6.1% | 14.3% | 11.1% | 0.0% |
Cases of infection were associated to an index case in 25% of Campylobacter cases and 32% of Salmonella cases. When an index case was identified (n=238), in cases of Campylobacter it was a sibling in 28.4% of cases, another relative in 51.5% and a child that attended the same school or childcare facility in 19.4%; in cases of Salmonella, it was a sibling in 38.2%, another relative in 44.9%, and a child attending the same school or childcare facility in 15.7% of cases.
Table 3 summarises the clinical manifestations of the main causative agents of diarrhoea. There were stool abnormalities in 72.9% of episodes (presence of mucus, blood, incompletely digested foods). Campylobacter was associated with blood in stools in 76.6% of the episodes with abnormal stools, compared to 69.7% for Salmonella.
Major signs and symptoms by aetiological agent (number and percentage of cases in which they were present by pathogen).
| Signs/symptoms | Campylobacter | Salmonella | Aeromonas | Yersinia | Coinfection |
|---|---|---|---|---|---|
| Fever | 316/434 (72.8%) | 170/232 (73.3%) | 10/20 (50%) | 15/18 (83.3%) | 4/11 (36.4%) |
| Vomiting | 169/430 (39.3%) | 102/230 (44.3%) | 7/20 (35%) | 8/18 (44.4%) | 7/11 (63.6%) |
| Abnormal stools | 319/429 (74.4%) | 162/222 (73%) | 13/20 (65%) | 11/15 (73.3%) | 8/11 (72.7%) |
The probability of having diarrhoea longer than one week was greater in cases of Salmonella compared to Campylobacter (55% vs 48%; OR, 1.33; 95% CI, 0.97–1.83). Diarrhoea resolved within one week in only 25% of cases caused by Aeromonas. The duration of diarrhoea exceeded 4 weeks in 5.6% of cases due to Yersinia, 3.2% of cases due to Campylobacter and 2.6% of cases due to Salmonella. The probability of admission to hospital was greater for patients with Salmonella compared to Campylobacter (10% vs 6%; OR, 1.67; 95% CI, 0.94–2.99). Ten percent of cases due to Aeromonas required hospital admission. The median length of stay was 3 days for Campylobacter (interquartile range, 2–4.25 days) and 2 days for Salmonella (interquartile range, 1–3.5 days). None of the patients in the series died.
The management of 33.2% of cases included antibiotherapy (32% of Campylobacter, 34.5% of Salmonella, 50% of Aeromonas and 33.3% of Yersinia cases). In patients with Campylobacter, 74.1% of prescriptions were for macrolides (54.7% azithromycin), 9.3% for trimetoprim/sulfamethoxazole and 6.5% for amoxicillin/clavulanic acid. In patients with Salmonella, 48.1% were for macrolides, 22.1% for trimetoprim/sulfamethoxazole, 14.3% for amoxicillin/clavulanic acid and 7.8% for combined antibiotherapy. In most cases the reason for prescribing antibiotherapy was persistence of symptoms (56%) or severity of disease (18%), while less frequent reasons included prescription by another provider (9%), epidemiological reasons (9%) or coinfection (5%).
The multivariate analysis—in which the presence of Campylobacter or Salmonella was the dependent variable and the independent variables were age less than 3 years, having siblings, school attendance, rural vs urban setting, fever, vomiting, abnormal stools, resolution within 1 week, hospital admission and antibiotic treatment—only found a significant association for a lower frequency of Salmonella compared to Campylobacter in children aged less than 3 years (adjusted OR, 0.61; 95% CI, 0.43–0.86; P=.005) and a greater frequency of Campylobacter in rural settings (adjusted OR, 1.48; 95% CI, 1.07–2.07; P=.012). The difference in the time elapsed to resolution, which was shorter in Campylobacter compared to Salmonella, approximated statistical significance (adjusted OR, 1.38; 95% CI, 0.99–1.92; P=.052).
In the analysis in which the dependent variable was whether patients received antibiotic treatment, logistic regression showed that there was a significantly greater probability of receiving it in cases with abnormal stools (adjusted OR, 1.53; 95% CI, 1.04–2.27; P=.031), with duration of diarrhoea of more than 7 days (adjusted OR, 2.81; 95% CI, 2.01–3.93; P<.000) or requiring hospital admission (adjusted OR, 1.95; 95% CI, 1.08–3.52; P=.027).
Since antibiotic susceptibility testing was performed in different microbiology laboratories, testing was not homogeneous. Table 4 presents the percentages of susceptibility and resistance to major antimicrobials found in different bacteria.
Percentage of strains susceptible, resistant or with intermediate resistance to major antibiotics based on susceptibility tests.
| Campylobacter (n=303) | Salmonella (n=204) | Aeromonas (n=16) | Yersinia (n=16) | Shigella (n=6) | |
|---|---|---|---|---|---|
| Antimicrobial susceptibility tests performed | 69.3% | 87.9% | 80% | 89.1% | 100% |
| Ampicillin | 40% S 60% R | 42% S 58% R | 100% R | 100% R | 80% S 20% R |
| Amoxicillin/clavulanic acid | 88% S 9% R 3% I | 87% S 8% R 5% I | 33% S 40% R 27% I | 23% S 77% R | 100% S |
| Cefotaxime | 82% S 18% I | 100% S | 85% S 15% I | 100% S | 100% S |
| Cefuroxime | 50% S 50% R | 73% S 27% R | 100% S | 50% S 50% R | 100% S |
| Ceftazidime | 100% S | 100% S | 100% S | ||
| Erythromycin | 97% S 3% R | 100% Sa | |||
| Azithromycin | 98% S 2% R | 100% Sa | |||
| Trimetoprim/sulfamethoxazole | 36% S 62% R 2% I | 95% S 5% R | 95% S 5% R | 87% S 3% R | 17% S 83% R |
| Fosfomycin | 92% S 8% I | 98% S 2% R | 100% S | ||
| Gentamicin | 98% S 2% R | 37% S 62% R 1% I | 100% S | 100% S | 100% S |
| Ciprofloxacin | 8% S 91% R 1% I | 92% S 5% R 3% I | 100% S | 100% S | 83% S 17% R |
I, intermediate resistance; R, complete resistance; S, susceptible.
Due to the decline in the prevalence of bacterial AGE in children, few recent studies have analysed its epidemiology or current approaches to its management in developed countries. Our study offers a perspective on its current situation and describes how Spanish PC paediatricians manage this disease. We could not infer its incidence, as patients are only evaluated when something suggests the possibility of a treatable bacterial disease, which usually occurs in patients with more severe presentations. We also cannot draw conclusions on the current situation and management of bacterial AGE in children with chronic diseases, as we excluded them on the basis that they would undergo specific evaluations.
The most recent clinical practice guidelines call for limiting performance of stool culture to specific situations1–7,9–12: chronic underlying disease (such as tumours, intestinal inflammatory disease or immunodeficiencies), extremely severe signs or symptoms, persistent symptoms (more than 7 days), outbreaks (childcare centres, schools or hospitals), severe bloody diarrhoea or past travel to high-risk regions. In our case series, most stool cultures were performed due to the presence of stool abnormalities and/or the duration or severity of symptoms.
The aetiological agents were consistent with those described for countries in our region. The data reported for Europe for year 2013 by the EFSA and the ECDC showed an organism distribution similar to the one in our study.13 The report of the SIM on samples from 201315,16 for children aged less than 15 years also presented isolation percentages (62.7% Campylobacter; 34.8% nontyphoid Salmonella) that were very similar to those found in our study (59.9% and 31.8%, respectively). Recently, a study conducted by Tam et al.17 in a similar environment (United Kingdom) found that the number of cases of viral AGE continues to increase, and that while cases of salmonellosis are declining, there is an increasing trend in AGE caused by Campylobacter.
Consistent with current data, cases were more frequent in boys and in early childhood (56.3% of patients aged less than 4 years), although there is a certain bias, as younger children, who usually have more severe symptoms, are assessed more thoroughly than older ones.
Although our findings are limited because the data were not complete (in fact, the suspected mechanism of transmission was not reported in 1 out of every 3 cases), person-to-person transmission was reported in most cases (70%). Poultry products (meat and eggs) continued to be the most frequent source in cases of suspected foodborne illness. Consequently, preventive measures should focus on reducing the possibility of transmission through an index case or through the processing and handling of poultry meats and eggs. The most effective measures are probably keeping sick children away from school and hand hygiene (mainly handwashing) in patients and contacts. Transmission occurs most often in the household or in school or childcare facilities.
Our analysis by AC, and comparing our data with the 2014 data of the SIM,15,16 found consistent results for the Canary Islands, with a greater frequency of nontyphoid Salmonella. The data of the SIM are not complete for Asturias and Galicia, and show a greater number of isolates of Campylobacter than of Salmonella in Castilla-La Mancha, contrary to what we found in our study. Data for other ACs were also consistent with our findings.18,19
Traditionally, AGE caused by Salmonella is considered to be more severe and clinically significant than AGE by Campylobacter. Our study confirmed that there were more hospital admissions due to salmonellosis (10% of cases) than to campylobacteriosis (6%), although the length of stay in days was shorter for Salmonella than for Campylobacter, which contrasts with the fact that for the overall cases for these two pathogens, the resolution of symptoms occurred earlier in cases of AGE by Campylobacter.
Despite the recommendations for antibiotic treatment in recent CPGs1–7,9–12 (only in infants aged <3 months, cases with severe symptoms or for epidemiological reasons, such as preventing the spread of outbreaks), 1 out of 3 cases of Salmonella and Campylobacter were treated with antibiotics. The reasons given for their use were mainly persistence of symptoms (56% of patients treated with antibiotics) and their severity (18%). The recommendations for Salmonella are to use antimicrobials in patients that are immunocompromised, aged less than 3 months (6 months for NICE), or with bacteraemia,2,7,20 and it seems that in primary care settings there is a tendency to use antibiotherapy if symptoms persist, even if the AGE is not severe. For Campylobacter, the fact that treatment early after onset is recommended to reduce its infectivity justifies the percentage of treated children, but “epidemiological reasons” were only reported for 9% of the treated cases. Macrolides were used in most of the cases, although the susceptibility of Salmonella to these drugs is not well defined, as the inclusion of macrolides in antimicrobial susceptibility tests is not recommended for this bacterium.21 Antimicrobial resistances in the bacteria under study followed the expected pattern, although we were unable to make comparisons between ACs because the 168 laboratories did not have uniform criteria.
The main limitations of our study are those that could make the data not be representative in a study conducted through a research network of national scope. We attempted to alleviate these by taking advantage of the network setup, as it comprehends the data of 288,800 children (4.1% of the Spanish population under 15 years). Since only 71% of the 304 sentinels collaborated in the study, it is possible that the sample is not representative enough, and this concern is particularly relevant for the 3 ACs for which we obtained the fewest questionnaires (Catalonia, Balearic Islands and Canary Islands). There are also the potential biases associated with surveys, such as noncoverage bias, nonresponse bias and interviewer bias. In order to minimise these biases, we made a pilot survey and provided clear and specific directions to sentinels regarding the inclusion of patients and data collection.
The main value of the findings of the study is that they provide an approximation to the actual management in primary care of AGE due to Campylobacter and Salmonella in Spanish children. The main strengths are the national scope of the study and the use of the network, which allows the accumulation of data for a substantial number of cases.
We conclude that the distribution of aetiological agents is characteristic of a developed country and that most transmissions were attributed to direct person-to-person contact rather than consumption of contaminated food. Furthermore, from what we found in the study, primary care paediatricians ought to adjust their practice to current recommendations, decreasing the number of cases of salmonellosis, mostly, but also of campylobacteriosis treated with antimicrobials, and using the appropriate antibiotic, when applicable, to treat Salmonella.
FundingThis study was supported by the Primary Care Paediatrics Epidemiologic Surveillance Network (Red de Vigilancia Epidemiológica en Pediatría de Atención Primaria [PAPenRED]), and, with no allocated funds, depended on the disinterested and generous contribution of each of the sentinel collaborators, autonomous community coordinators and national coordinators.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: García Vera C, García Ventura M, del Castillo Aguas G, Domínguez Aurrecoechea B, Esparza Olcina MJ, Martínez Rubio A, et al. Gastroenteritis aguda bacteriana: 729 casos reclutados por una red nacional de atención primaria. An Pediatr (Barc). 2017;87:128–134.
The findings of this study have been accepted for presentation in oral communication and poster form at the 64 Congress of the Asociación Española de Pediatría.