Despite the potential risks of drug use during pregnancy, consumption has increased in recent decades.
ObjectiveTo identify the risk of congenital anomalies (CA) associated with the use of drugs in primary care in pregnant women resident in the Valencia Region.
MethodsA case-control study, considering a case as a less than one year old live birth in 2009–2010, diagnosed with a CA and resident in the Valencia Region, obtained from the CA population-based registry. Controls were selected from the Metabolic Disease Registry, and the drugs prescribed and dispensed from the Integral Management of Pharmaceutical Services. Crude odds ratio (OR) was calculated with its 95% confidence intervals and adjusted OR was calculated using logistic regression.
ResultsA total of 1.913 cases and 3.826 controls were identified. The most frequently used drug groups were those acting on the musculoskeletal, nervous and respiratory systems, on the blood and blood forming organs, and anti-infection drugs. The most common drugs used were ibuprofen, dexketoprofen, paracetamol, amoxicillin, ferrous sulphate, and a combination of folic acid. A significantly increased risk of CA was identified for drugs acting on the musculoskeletal system (adjusted OR 1.14 [95% confidence interval 1.02–1.28]). A significantly decreased risk was observed for drugs acting on the blood and blood forming organs (adjusted OR 0.87 [95% confidence interval 0.78–0.98]).
ConclusionsAssociations between drugs and CA in pregnant women resident in the Valencia Region have been identified for drugs that act as risk factors of CA, and for drugs that act as protective factors of CA.
El consumo de medicamentos durante el embarazo se ha incrementado en las últimas décadas.
ObjetivoIdentificar el riesgo de anomalías congénitas (AC) asociado a la utilización de medicamentos en atención ambulatoria en embarazadas residentes en la Comunitat Valenciana.
MétodosEstudio de casos-controles, considerando caso a menores de un año nacidos vivos en 2009-2010 diagnosticados de AC y residentes en la Comunitat Valenciana, obtenidos del registro poblacional de AC. Los controles se seleccionaron del Registro de Metabolopatías y la medicación prescrita y dispensada se obtuvo del módulo Gestión Integral de Prestación Farmacéutica. Se calcularon las odds ratio (OR) y los intervalos de confianza al 95% y las OR ajustadas mediante regresión logística.
ResultadosSe identificaron 1.913 casos y 3.826 controles. Los grupos de medicamentos más frecuentemente prescritos y dispensados fueron: los que actúan sobre los sistemas musculoesquelético, nervioso, respiratorio, sobre la sangre y órganos hematopoyéticos, y antiinfecciosos. Los medicamentos más habituales fueron: ibuprofeno, dexketoprofeno, paracetamol, amoxicilina, sulfato de hierro y una combinación de ácido fólico. Se identificó un aumento del riesgo de anomalías congénitas significativo para los fármacos de acción sobre el sistema musculoesquelético (OR ajustada de 1,14 [intervalo de confianza al 95% 1,02-1,28]). Se observó una disminución del riesgo significativa en el grupo que actúa sobre la sangre y los órganos hematopoyéticos (OR ajustada de 0,87 [intervalo de confianza al 95% 0,78-0,98]).
ConclusionesSe han identificado asociaciones de medicamentos con AC en mujeres embarazadas residentes en la Comunitat Valenciana, tanto para fármacos que actúan como factores de riesgo de AC como para fármacos que actúan como factores protectores de AC.
The term congenital anomaly (CA) comprehends any type of defect in physical, psychological, functional, sensory or motor development that occurs during intrauterine life and is detected during pregnancy, labour or later in life. It may even include genetic disorders and inborn errors of metabolism.1
Congenital anomalies are an important public health problem on account of their impact on the quality of life of affected patients and families, their contribution to foetal and infant mortality, emotional costs to the family, and the financial cost of medical, social and educational services to improve quality of life of affected individuals and their families.2
The aetiology of CAs in unknown; it is believed that they result from interactions between genetic and environmental factors, although their specific interactions and relative importance have not yet been established.3,4
Since the teratogenic effect of thalidomide was first discovered,5 there have been advances in the investigation of the teratogenic effects that specific drugs may have on the foetus through a variety of mechanisms, especially when used in the first trimester of pregnancy. However, the results obtained by these studies have not always been conclusive.6 Thus, drugs used for the treatment of hyperthyroidism, such as carbimazole or thiamazole, have been associated with CAs like choanal atresia and omphalocoele,7 although this association has not been observed in every study.8
Antibiotic drugs have been studied extensively and shown to have different effects. Penicillins, erythromycin and cephalosporins have not been associated to CAs. Cleft lip and palate have been associated with the use of amoxicillin in the early months of pregnancy. Sulfamides and nitrofurantoins have been associated with severe CAs such as anencephaly and cardiac CAs, as well as choanal atresia, lip and cleft palate and diaphragmatic hernia.9,10
Findings for other drug classes such as antiepileptic drugs have been contradictory11,12 or the evidence of their association with CAs was inconclusive.13 In other instances, it was difficult to differentiate between potential teratogenic effects of drugs and the effects of the underlying disease.14,15
Despite the potential risks of using medications during pregnancy, especially in the first trimester, consumption has increased by more than 60% in recent decades, and at least one drug is used in 50% of pregnancies.16 Furthermore, potentially teratogenic drugs are sometimes prescribed without appropriate guidance on contraception.17 It is estimated that 1% of pregnant women are exposed to potentially teratogenic drugs, with an estimated proportion of 0.6% for the first trimester.18
In the Autonomous Community of Valencia (ACV) there is a module for the integral management of pharmaceutical services (Gestión Integral de Prestación Farmacéutica [GAIA])19 within the outpatient care system electronic database (Sistema de Información de la Asistencia Ambulatoria) that can be used to study the use of pharmaceuticals, as it records the drugs prescribed by clinicians in outpatient clinics, allowing for future data collection.
In addition, a population register of CAs in the ACV has been created recently. The register includes cases of CAs in infants aged less than 1 year residing in the ACV, diagnosed according to the criteria and quality control standards of the European Network of Population-Based Registries for the European Surveillance of Congenital Anomalies20 (EUROCAT), and makes available validated data on CAs in the ACV. There is also the Register of Metabolic Disorders of the ACV, which has records of the births in the autonomous community every year because newborns are routinely screened for metabolic disorders, and has information on newborns that are not included in the population register of the ACV.
The aim of our study was to identify pregnant women residing in the ACV with a potential risk of CAs associated to the prescription and dispensation of drugs in outpatient care settings as indicators of pharmaceutical use during pregnancy.
Materials and methodsWe conducted an epidemiological case-control study by collecting retrospective data from secondary sources.
We defined case as an infant aged less than 1 year born alive between 2009 and 2010 and residing in the ACV who received a diagnosis of CA (EUROCAT definition20) in the first year of life, and control as an infant born alive in the 2009–2010 period and residing in the ACV who did not receive a diagnosis of CA in the first year of life. We randomly selected 2 controls per case (matching controls by sex, month and year of birth, and province of residence).
We collected the following data on live births: health care card number, month and year of birth, multiparity, type of delivery, birth weight and gestational age, and type of CA (for cases). We also collected the following for mothers: health care card number, age and municipality of residence at time of delivery, country of birth, and medication prescribed and received during pregnancy.
We obtained data from the following sources: CA population register (selection of cases and collection of data for variables under study, collection of maternal data save for medication), Metabolic Disorder Register (selection of controls and collection of data for infants and mothers—save for medication received by mothers) and GAIA (collection of data on medication prescribed and dispensed in outpatient care to mothers of both cases and controls).
We were able to integrate the information from the different sources and identify the mother of each infant using the health care card numbers. The health care card number of the mother was encrypted in the GAIA database, so we obtained the medication data by encrypting the health care card number of the mothers of cases and controls using the same algorithm. The patients were not identifiable in the statistical analysis, as we used the Stata 12 software21 to irreversibly anonymise the data in the final database created for the study.
We conducted a descriptive study of the drugs prescribed and dispensed to the mothers of cases as well as controls. To do so, we defined the exposure period as the interval ranging from the 30 days preceding the estimated date of conception to birth. We included the 30 preceding days to ensure that the exposure period covered the entire pregnancy. We calculated the date of conception by subtracting the weeks of gestation from the date of birth. We coded and grouped the prescribed and dispensed pharmaceuticals using the Anatomical Therapeutic Chemical classification.22 We considered the exposure of a mother to each individual pharmaceutical separately. We described pharmaceuticals by group, identifying the most frequently prescribed and dispensed drugs in cases and controls.
Using the data on place of residence, we analysed potential differences in prescription and dispensation of drug groups between the different health areas. We identified significant differences by means of Pearson's chi square test. We analysed cases and controls separately.
We performed an exploratory bivariate analysis to detect associations between each drug group and the most frequently prescribed and dispensed drugs in cases and controls. We calculated crude odd ratios (ORs) with their 95% confidence intervals (CIs). We included the drugs or drug groups for which we found significant differences in the multivariate analysis.
We fitted logistic regression models to calculate adjusted ORs. We adjusted the model for the following variables, which have been previously described as confounders in the literature: maternal age (<20, 20–35, >35 years), birth weight (≥2500g, <2500g), gestational age (≥37, <37 weeks gestation), multiparity (no/yes), maternal country of origin (born in/outside of Spain) and health area (1 through 23). We performed regression diagnostics to assess the colinearity of independent variables (using the variance inflation factor). We performed analyses for CAs overall and for each group of anomalies.
ResultsWe identified a total of 1.913 infants born alive between 2009 and 2010 and residing in the ACV that received a diagnosis of CA in the first year of life. We selected 3.826 controls born alive and residing in the ACV that did not receive a diagnosis of CA in the first year of life. Table 1 summarises the characteristics of these newborns and their mothers by membership in the case or control group.
Characteristics of live births and mothers by study group.
| Controls | Cases | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Live births | ||||
| Year of birth | ||||
| 2009 | 1948 | (50.9) | 974 | (50.9) |
| 2010 | 1878 | (49.1) | 939 | (49.1) |
| Sex | ||||
| Male | 1957 | (51.2) | 1058 | (55.3) |
| Unknown | 0 | (0.0) | 1 | (0.1) |
| Multiparity | ||||
| Yes | 141 | (3.7) | 126 | (6.6) |
| Mother | ||||
| Age, years | ||||
| <20 | 106 | (2.8) | 72 | (3.8) |
| 20–35 | 2872 | (75.1) | 1304 | (68.2) |
| >35 | 844 | (22.1) | 505 | (26.4) |
| Unknown | 4 | (0.1) | 32 | (1.7) |
| Province | ||||
| Valencia | 1920 | (50.2) | 862 | (45.1) |
| Alicante | 1443 | (37.7) | 804 | (42.0) |
| Castellón | 459 | (12.0) | 246 | (12.9) |
| Unknown | 4 | (0.1) | 1 | (0.1) |
| Country | ||||
| Spain | 2818 | (73.7) | 1340 | (70.0) |
| Not Spain | 968 | (25.3) | 524 | (27.4) |
| Unknown | 40 | (1.0) | 49 | (2.6) |
Of the 27.712 prescriptions that were dispensed, 33.2% corresponded to mothers of cases and 66.8% to mothers of controls. We found medication data for 14.0% of the mothers of cases and 15.1% of mothers of controls.
The most frequent drug groups were the same in cases and controls: drugs that act on the respiratory, nervous and musculoskeletal systems, blood and blood-forming organs and antiinfectives.
We only found changes in the proportion in the antiinfectives group, which rose from fourth position in prescription to being the most frequently dispensed group. The blood and blood-forming organs drug group and the musculoskeletal drug group switched places when we compared cases and controls, with a higher proportion of the former in controls and a higher proportion of the latter in cases.
The most frequent individual drugs in both cases and controls were ibuprofen, ferrous sulfate, amoxicillin, folic acid in combination with potassium iodide and cyanocobalamin, paracetamol and dexketoprofen.
Ibuprofen was the most frequently prescribed and dispensed drug in both cases and controls. We found some difference in the prescription and dispensation proportions of some drugs; for instance, the second most frequent drug was ferrous sulfate when it came to prescription (3.0%) but it was amoxicillin when it came to dispensation (3.0%). We did not find differences between cases and controls when we compared prescription and dispensation separately.
We analysed patterns in prescription and dispensation by health area, and only found significant differences in the case group: differences in the prescription and in the dispensation of drugs in the group acting on the genitourinary system and the sex hormones (P=.008 and P<.001, respectively), and differences in the dispensation of drugs in the respiratory system group (P=.03).
We obtained the proportion of prescriptions and of dispensations per 100000 inhabitants in the different health areas for cases and for controls. In the case group, the health areas with the highest proportions per total inhabitants were the same and ranked in the same order for prescriptions and for dispensations. The same occurred in the control group, although the health areas were different.
The risk of CAs associated to the prescription or the dispensation of drugs overall was not significant.
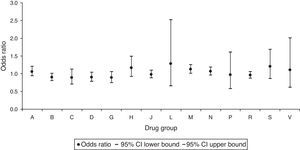
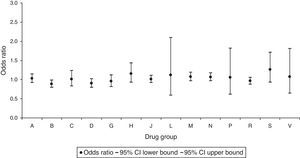
When we analysed the risk associated with prescription by drug group, we found that there was a statistically significant increase in the risk of CAs for the group of drugs acting on the musculoskeletal system, with an OR of 1.13 (95% CI, 1.01–1.26) (Fig. 1). We also observed a protective effect against CAs in the dispensation of the group of drugs that act on blood and blood-forming organs, with an OR of 0.88 (95% CI, 0.79–0.99) (Fig. 2).
Odd ratios for congenital anomalies per drug group (drug prescription).
A, alimentary tract and metabolism; B, blood and blood-forming organs; C, cardiovascular system; CI, confidence interval; D, dermatologicals; G, genitourinary system and sex hormones; H, systemic hormonal preparations; J, antiinfectives for systemic use; L, antineoplastic and immunomodulating agents; M, musculoskeletal system; N, nervous system; P, antiparasitic products, insecticides and repellents; R, respiratory system; S, sensory organs; V, various.
Odd ratios for congenital anomalies per drug group (drug dispensation).
A, alimentary tract and metabolism; B, blood and blood-forming organs; C, cardiovascular system; CI, confidence interval; D: dermatologicals; G, genitourinary system and sex hormones; H, systemic hormonal preparations; J, antiinfectives for systemic use; L, antineoplastic and immunomodulating agents; M, musculoskeletal system; N, nervous system; P, antiparasitic products, insecticides and repellents; R, respiratory system; S, sensory organs; V, various.
The risk of CAs was also not significant for any of the most frequently prescribed or the most frequently dispensed drugs.
Lastly, we performed a multivariate analysis of the 2 drug groups in which we have found statistically significant differences in cases and controls. We adjusted the OR by birth weight and gestational age, multiparity, maternal age, health area, and maternal country of origin. When we assessed the colinearity of the independent variables we obtained a variance inflation factor of less than 3 in every instance.
The statistically significant increase in risk associated to the prescription of the musculoskeletal system drug group was maintained, with an adjusted OR of 1.14 (95% CI, 1.02–1.28), as was the significant decrease in risk of CAs associated with the dispensation of drugs in the blood and blood-forming organs group, with an adjusted OR of 0.87 (95% CI, 0.78–0.98).
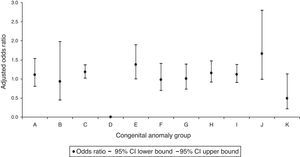
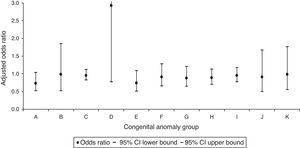
In the analysis by CA groups, we only identified a significant increase in the CAs of the circulatory system with an adjusted OR of 1.18 (95% CI, 1.01–1.37) associated to the prescription of drugs that act on the musculoskeletal system (Fig. 3). We did not find a significant association of any specific CA group with the dispensation of drugs that act on the blood and blood-forming organs (Fig. 4).
Adjusted odd ratios for congenital anomalies per group of congenital anomalies in the prescription of drugs acting on the musculoskeletal system.
A, nervous system; B, eye, ear, face and neck; C, circulatory system; CI, confidence interval; D, respiratory system; E, cleft lip and palate; F, digestive system; G, genital organs; H, urinary system; I, musculoskeletal system; J, other malformations; K: chromosomal abnormalities.
Adjusted odd ratios for congenital anomalies per group of congenital anomalies in the prescription of drugs acting on blood and blood-forming organs.
A, nervous system; B, eye, ear, face and neck; C, circulatory system; CI, confidence interval; D, respiratory system; E, cleft lip and palate; F, digestive system; G, genital organs; H, urinary system; I, musculoskeletal system; J, other malformations; K: chromosomal abnormalities.
Pharmaceutical use was more common in controls, with 15% of mothers consuming pharmaceuticals during pregnancy compared to 14% of mothers in the case group. These figures are considerably lower than those described in other studies, with proportions ranging widely in the literature (from 37% to 81%).16,23,24
The frequent use of drugs in pregnant women is mostly due to their use being unavoidable in many instances. There are situations in which medication is necessary and the benefits outweigh the associated teratogenic risk.25 The key interventions in the approach to such situations are to assess the risk and benefits of the drug before prescription, administer the minimum effective dose for the shortest possible time, use the least number of pharmaceuticals possible, and select drugs that have been widely used in clinical practice. This assessment needs to be performed in each individual pregnancy, and the use of drugs in the first trimester of pregnancy should be avoided.26
The drugs used most frequently in our study were consistent with those identified as most common in other studies,24,27 and there are two possible categories: those recommended for use during pregnancy, and those used to treat conditions that arise during gestation.
Our analysis of the differences between health departments in the ACV did not lead to the identification of any geographical patterns. In the future, it would be advisable to design studies capable of identifying any existing geographical differences in the use of medications and their causes, as the international literature has described significant geographical variability in drug use,28 and there is also evidence of geographical patterns in the prevalence and distribution of some CAs in the ACV.29
The prescription to pregnant women of drugs that act on the musculoskeletal system, especially of 2 nonsteroidal anti-inflammatory drugs (NSAIDs), ibuprofen and dexketoprofen, was associated with a significant increase in the risk of CAs in the child (adjusted OR, 1.14) and specifically in the risk of CAs of the circulatory system (adjusted OR, 1.18). This was consistent with other studies in which the adjusted ORs for CAs overall and for cardiac CAs revealed a deleterious association with the use of NSAIDs,30 although there are also studies that have not found evidence of this association.31
One possible explanation for the increased risk of CAs detected in our study is that one of the most common foetal adverse effects of NSAIDs is the premature closure of the ductus arteriosus, leading to right ventricle overload, hypertrophy and dilatation, which in turn are associated with tricuspid regurgitation and a right-to-left shunting.32 Furthermore, decreased foetal renal artery flow and oligohydramnios may recur when nonsteroidal anti-inflammatory therapy is discontinued,33 which can cause pulmonary hypertension and is associated with increased cutaneous and intracranial bleeding in the foetus, and protracted labour, increased blood loss and increased severity of anaemia in the mother.34
Another salient finding involved the dispensation of drugs that act on blood and blood-forming organs (especially ferrous sulfate and the combination of folic acid, potassium iodide and cyanocobalamin), which was associated with a significantly decreased risk of CAs in the offspring (adjusted OR, 0.87). We did not identify any protective effects on specific groups of CAs, although there is ample evidence on the protective effect of folic acid combined with a multivitamin against the development of neural tube CAs35 and other groups of malformations.36 Furthermore, iron is essential for the prevention of anaemia, which delays foetal growth during gestation and increases the risk of miscarriage, genetic and nervous system malformations, low birth weight and premature birth.37
Since we conducted a population-based case-control study by the collection of retrospective information from secondary sources, we minimised the risk of selection bias inherent in sample-based studies as well as the differential misclassification bias inherent in non-population-based surveys of cases and controls. We also minimised nonresponse bias.
One of the limitations of our study was the quality of the data from secondary sources. When it came to the population register of CAs, the health care card number of the mother was missing in 13.1% of entries, so we were unable to integrate any corresponding data on pharmaceuticals that may have been available for those cases. As for GAIA, the prescription and dispensation of drugs is underrecorded, a drawback already mentioned by other authors,38,39 since the system does not include prescriptions for drugs that are not paid by the Spanish public health system, over-the-counter drugs or drugs prescribed by private physicians. Furthermore, in the case of inexpensive drugs, consumers may prefer to buy them directly rather than going through the process of obtaining a prescription. This type of underrecording is improbable in more expensive drugs, drugs that may require dose adjustments or drugs that require medical monitoring.38 Nevertheless, this database, despite its limitations, allowed us to make an estimation of pharmaceutical use in the ACV that would have been far more costly had we used other methods.
Another limitation is that we used the prescription and dispensation of drugs recorded in GAIA as a proxy for drug use in pregnant women, and that we could not break down drug exposure by trimester of pregnancy. In addition, the design of the study did not allow us to determine with certainty that pharmaceuticals were involved in the aetiology of CAs, but we were able to establish the risk associated to their use. Their actual contribution to CAs could only be determined by taking into account other possible etiological factors (environmental, genetic, socioeconomic, etc.).
However, data on prescribed and dispensed drugs during pregnancy can be used as an approximation of drug use by pregnant women. The availability of data on drug exposure can help identify drugs that are potentially teratogenic and establish the association of drugs with CAs as protective or risk factors. Furthermore, this can lay the foundation for the development of clinical and administrative practice guidelines, allowing the implementation of customised pharmacological treatments that are effective and safe during pregnancy, and for the systematic surveillance of the risk of CAs associated with drug exposure using these source data.
FundingThis study was funded by the Instituto Médico Valenciano, the project funded by the Fondo de Investigaciones Sanitarias (FIS PI10/01676) and the Spanish Rare Diseases Registries Research Network (SpainRDR) and had the support of the International Rare Diseases Research Consortium (IRDiRC) and the Instituto de Salud Carlos III (PR11/122).
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Cavero-Carbonell C, Gimeno-Martos S, Páramo-Rodríguez L, Rabanaque-Hernández MJ, Martos-Jiménez C, Zurriaga Ó. Consumo de medicamentos en el embarazo y riesgo de anomalías congénitas en la Comunitat Valenciana. An Pediatr (Barc). 2017;87:135–142.
Previous presentations: this study was presented at the European Conference on the Safety of Medication use in Pregnancy, EUROmediCAT; February 2015; Poznan (Poland); and the I Congreso de Biomedicina Predocs de Valencia; November 2014; Valencia (Spain).