Norovirus is the second cause of acute viral gastroenteritis in infants after rotavirus. However, its prevalence is underestimated because a specific diagnosis is not usually performed. The comparative study of microbiological diagnostics, performed before and after the implementation date of a test for detecting a particular microorganism, allows the estimation of the percentage of cases not properly diagnosed earlier (for non-implementation of the test) and those that would be left to diagnose if the test is removed. In this paper we study the epidemiology of acute gastroenteritis virus before and after the implantation of the Norovirus GI+GII CerTest.
Material and methodsAn observational retrospective cohort study was conducted on patients under 15 years old with acute gastroenteritis, from January 2013 to April 2015. The sample was divided into two groups. In the first group, the search was limited to adenovirus and rotavirus, and in the second one, the determination of norovirus became part of the systematic diagnosis. The study included 604 patients, 313 in the first group and 291 in the second one.
ResultsDemographic characteristics were similar in both groups. In the first group, 58/313 (18.5%) enteric viruses were identified and in the second group, 97/291 (33.3%). In the second group, 31 positive cases for norovirus were identified, but only 12 (4.1%) of them were positive exclusively for this virus. No significant differences were found in clinical features of intestinal viruses.
ConclusionsAn actual increase of 4.1% was observed in the cases with an identified aetiological agent after implementing the Norovirus GI+GII CerTest diagnostic technique. The most common cause of acute gastroenteritis is rotavirus, closely followed by norovirus.
El norovirus es el segundo agente causal de las gastroenteritis agudas víricas en niños después del rotavirus. Su prevalencia está subestimada debido a que no se realiza habitualmente un diagnóstico específico. El estudio de los diagnósticos microbiológicos, realizados antes y después de la fecha de implantación de un test de detección de un microorganismo concreto, permite estimar el porcentaje de casos no diagnosticados con anterioridad (por la no implantación) y los que se dejarían de diagnosticar en caso de su supresión. En este artículo estudiamos la epidemiología de las gastroenteritis agudas por virus antes y después de la implantación del test CerTest Norovirus GI+GII.
Material y métodosEstudio observacional de cohortes retrospectivo realizado en pacientes menores de 15 años con gastroenteritis aguda desde enero de 2013 hasta abril de 2015. Se dividió la muestra en 2 grupos; en el primero la búsqueda se limitó a adenovirus y rotavirus y en el segundo la determinación de norovirus se incorporó al diagnóstico sistemático. Se incluyó a 604 pacientes, 313 en el primer grupo y 291 en el segundo.
ResultadosLas características demográficas fueron similares en ambos grupos. Se identificaron 58/313 (18,5%) virus entéricos en el primer grupo y 97/291 (33,3%) en el segundo. Del segundo grupo 31 muestras fueron positivas para norovirus, siendo 12 (4,1%) positivas exclusivamente para norovirus. No se encontraron diferencias significativas en las características clínicas de los virus intestinales.
ConclusionesSe observó un aumento real del 4,1% en el porcentaje de casos con agente etiológico identificado al implementar la técnica diagnóstica CerTest Norovirus GI+GII. El rotavirus sigue siendo la causa más frecuente de gastroenteritis aguda en nuestro medio, seguido de cerca por el norovirus.
Gastrointestinal infection is the second most common infectious disease in the paediatric population despite the improvements in public health infrastructures made in the past few decades.1
The WHO defines acute gastroenteritis (AGE) as a decrease in the consistency of stools or an increase in the frequency of evacuations (3 or more in 24h) with or without fever or vomiting and lasting fewer than 14 days. In infants, decreased stool consistency compared to previous faeces is more indicative of diarrhoea than stool number.2 It is the second leading cause of death in children aged less than 5 years.3 The incidence of AGE in healthy children in Europe is of approximately 0.5–2 episodes per child per year in children aged less than 3 years.2 In developing countries, this figure rises to 6 episodes per year.4 The burden of AGE is also substantial.5
When it comes to viral gastroenteritis, there are 4 major causative agents: rotavirus, adenovirus, norovirus and astrovirus.6 Rotavirus is the leading causative agent of AGE in children, accounting for more than half a million deaths and more than two million hospitalisations a year worldwide.2,7 However, in countries with a high vaccination coverage for rotavirus, norovirus is becoming a leading cause of gastroenteritis.2,8,9
Norovirus is an emerging pathogen whose prevalence is underestimated because there are many cases in which a specific diagnosis is never made. By studying the microbiological diagnoses made before and after the introduction of a test for detecting a specific microbe, it is possible to estimate the percentage of cases that were not diagnosed correctly in the past (for lack of testing) and the percentage that would no longer be diagnosed if the test was not performed, and therefore the reduction in the proportion of cases of idiopathic AGE. This is particularly relevant in the case of norovirus, for which a vaccine is currently being developed.10
In this article, we analyse the epidemiology of acute viral gastroenteritis before and after the introduction of the CerTest Norovirus GI+GII test, which allowed us to make the comparisons mentioned above.
Materials and methodsWe conducted a retrospective observational cohort study of patients with AGE aged less than 15 years in a tertiary level hospital and the primary care centres of the corresponding health district. The period of recruitment was January 2013 to April 2015.
The methods used for viral detection changed over the study period due to the introduction of new techniques and reagents in the market. We divided the sample into 2 groups. In the first group, tests for viral detection were limited to adenovirus and rotavirus. In the second group (starting from April 2014, except for specimens in which the amount of reagent was insufficient) testing for norovirus was added to the routine diagnostic workup. The method used for detection of rotavirus and adenovirus was the CerTest Rotavirus-Adenovirus combo card (CerTest Biotec),11 and the one used for the detection of norovirus was the CerTest Norovirus GI+GII combo card (CerTest Biotec).
We collected data from the electronic health records of patients that sought care for AGE in primary care centres and the emergency department and of patients admitted to hospital in whom a stool sample was collected for detection of enteric viruses (adenovirus, rotavirus and/or norovirus). We excluded patients in whom stool samples were collected that had sought care for reasons other than AGE (abdominal pain, rectal bleeding, suspected cow's milk protein allergy, etc.). We also excluded data for stool samples collected during the followup after resolution of AGE, and for patients in whom stool analysis was requested but the reason for testing or the presenting symptoms were not documented in the patient's chart.
We analysed data for a total of 604 samples. The minimum sample size required to detect significant differences in the detection of gastrointestinal viruses with an effect size of 10%, assuming a proportion of positive detections of 20% with the use of 2 diagnostic tests and of 30% with the use of all 3 tests, with a 95% confidence interval and a power of 80%, would be of 586 samples (293 per group).
We performed a descriptive analysis of all the variables under study, expressing quantitative variables as mean±standard deviation and qualitative variables as absolute frequencies, percentages and 95% confidence intervals. We analysed the association between qualitative variables with the chi square test or Fisher's exact test. We compared means with the Mann–Whitney U or Kruskal–Wallis test based on the number of groups being compared after verifying the normality assumption by means of the Kolmogorov–Smirnov test. We performed the statistical analysis with the SPSS 19.0 software. We defined statistical significance as a p-value of less than 0.05. The study protocol was approved by the competent clinical research ethics committee.
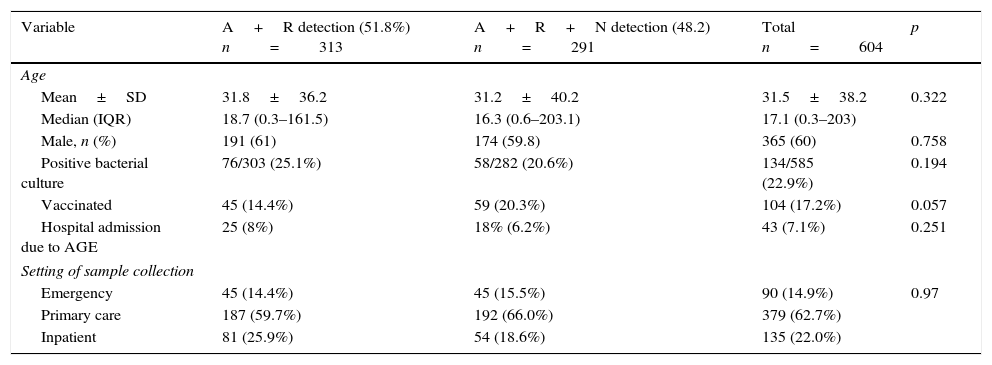
ResultsWe included 604 samples from patients that were divided into 2 groups. The diagnostic method used in the first group, which included 313 samples (51.8%; 95% CI, 47.7%–55.8%), tested for rotavirus and adenovirus, while the diagnostic test used in the second group, with 291 samples (48.2%; 95% CI, 44.1%–52.2%), included rotavirus, adenovirus and norovirus. The two groups had comparable characteristics, summarised in Table 1 (comparing both groups).
Demographic characteristics of both groups.
| Variable | A+R detection (51.8%) n=313 | A+R+N detection (48.2) n=291 | Total n=604 | p |
|---|---|---|---|---|
| Age | ||||
| Mean±SD | 31.8±36.2 | 31.2±40.2 | 31.5±38.2 | 0.322 |
| Median (IQR) | 18.7 (0.3–161.5) | 16.3 (0.6–203.1) | 17.1 (0.3–203) | |
| Male, n (%) | 191 (61) | 174 (59.8) | 365 (60) | 0.758 |
| Positive bacterial culture | 76/303 (25.1%) | 58/282 (20.6%) | 134/585 (22.9%) | 0.194 |
| Vaccinated | 45 (14.4%) | 59 (20.3%) | 104 (17.2%) | 0.057 |
| Hospital admission due to AGE | 25 (8%) | 18% (6.2%) | 43 (7.1%) | 0.251 |
| Setting of sample collection | ||||
| Emergency | 45 (14.4%) | 45 (15.5%) | 90 (14.9%) | 0.97 |
| Primary care | 187 (59.7%) | 192 (66.0%) | 379 (62.7%) | |
| Inpatient | 81 (25.9%) | 54 (18.6%) | 135 (22.0%) | |
A, adenovirus; AGE, acute gastroenteritis; IQR, interquartile range; N, norovirus; R, rotavirus SD, standard deviation.
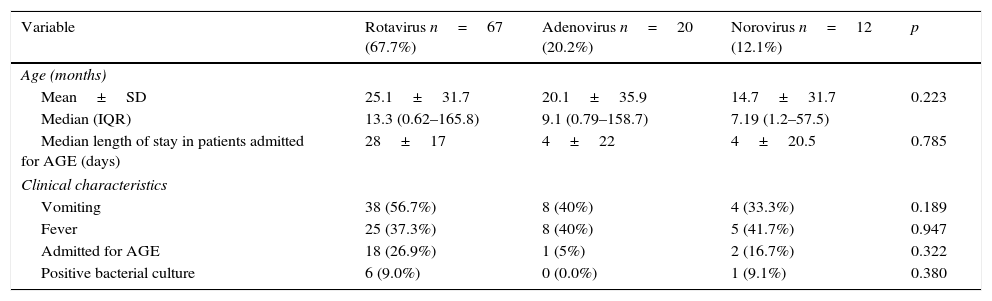
In the first group, 50 samples tested positive for rotavirus (16.0%; 95% CI, 11.8%–20.2%) and 8 to adenovirus (2.6%; 95% CI, 0.6%–4.4%). Of all the samples that tested positive for adenovirus or rotavirus, 3 (1.0%, 95% CI, 0.2%–2.8%) were positive to both. In the second group, rotavirus was detected in 33 samples (11.3%; 95% CI, 7.5%–15.1%); adenovirus in 33 (11.3%; 95% CI, 7.5%–15.1%) and norovirus in 31 (10.7%; 95% CI, 7.0%–14.4%). Of all these positive cases, 19 (2.7%; 95% CI, 0.7%–4.8%) were positive for more than 1virus. In total, the number of positive enteric virus detections was 58 out of the 313 samples in the first group (18.5%; 95% CI, 14.1%–23.0%) and 97 out of 291 samples in the second group (33.3%; 95% CI, 27.7%–38.9%). Table 2 describes the clinical characteristics of the samples positive to each of the viruses under study (rotavirus, adenovirus and norovirus), excluding samples positive for more than one virus.
Clinical characteristics of enteric viruses.
| Variable | Rotavirus n=67 (67.7%) | Adenovirus n=20 (20.2%) | Norovirus n=12 (12.1%) | p |
|---|---|---|---|---|
| Age (months) | ||||
| Mean±SD | 25.1±31.7 | 20.1±35.9 | 14.7±31.7 | 0.223 |
| Median (IQR) | 13.3 (0.62–165.8) | 9.1 (0.79–158.7) | 7.19 (1.2–57.5) | |
| Median length of stay in patients admitted for AGE (days) | 28±17 | 4±22 | 4±20.5 | 0.785 |
| Clinical characteristics | ||||
| Vomiting | 38 (56.7%) | 8 (40%) | 4 (33.3%) | 0.189 |
| Fever | 25 (37.3%) | 8 (40%) | 5 (41.7%) | 0.947 |
| Admitted for AGE | 18 (26.9%) | 1 (5%) | 2 (16.7%) | 0.322 |
| Positive bacterial culture | 6 (9.0%) | 0 (0.0%) | 1 (9.1%) | 0.380 |
AGE, acute gastroenteritis; IQR, interquartile range; SD, standard deviation.
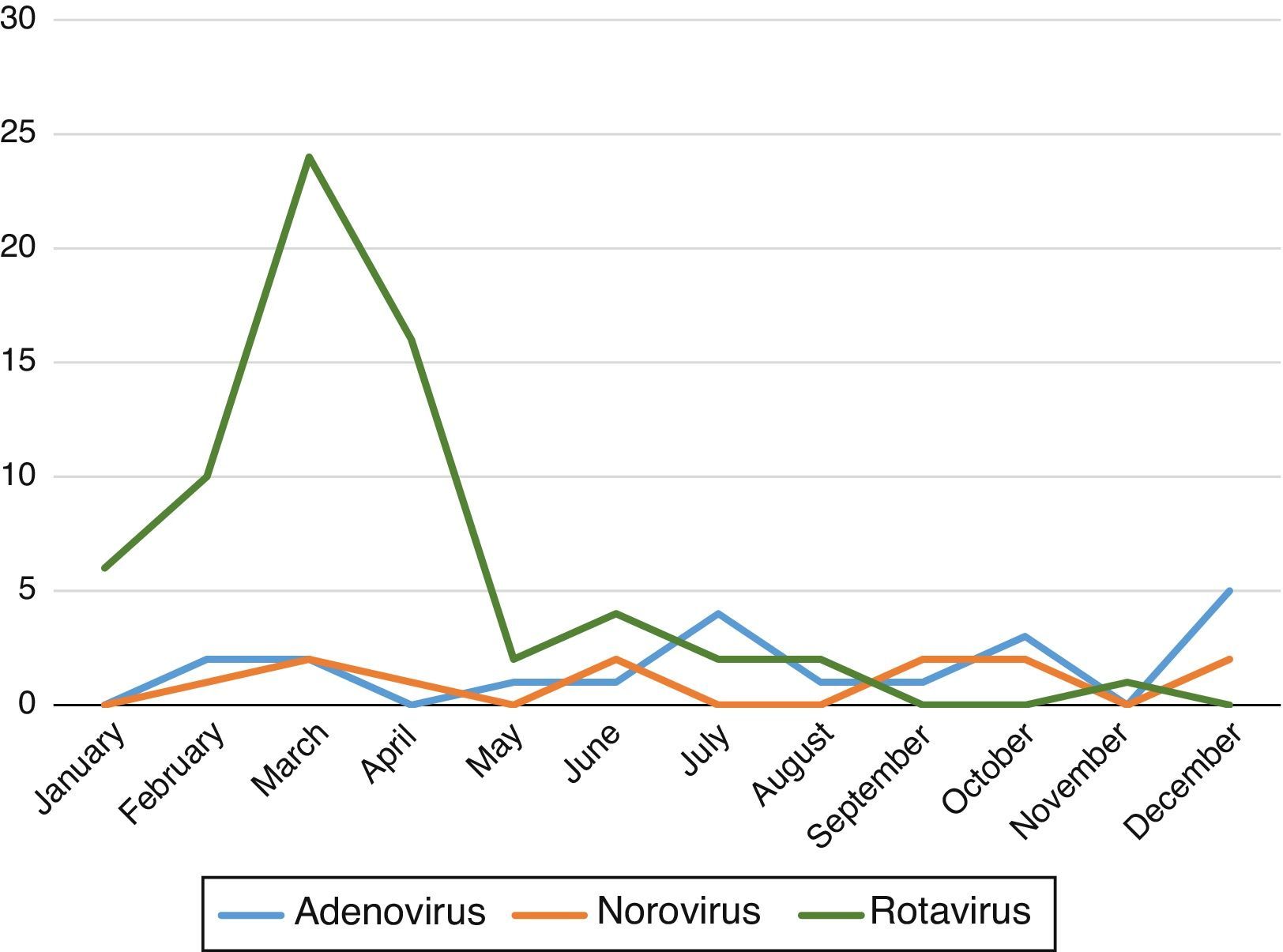
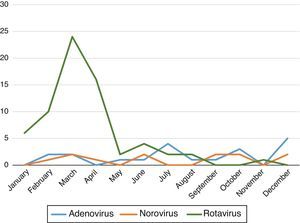
We found that the prevalence of AGE with detection of viruses in stool samples peaked in the early months of the year (January–April) with a second, mild peak in autumn (October–November). Fig. 1 shows the variations in the prevalence of the different viruses during the year.
Of the samples that tested positive for norovirus, 8 were also positive to adenovirus (25.8%; 95% CI, 8.8%–42.8%), 3 to rotavirus (9.7%; 95% CI, 2.0%–25.7%) and 8 were positive to all 3 viruses (25.8%; 95% CI, 8.8%–42.8%). Only 12 samples (4.1% of the total of patients included in the second period; 95% CI, 1.7%–6.6%) were only positive for norovirus. When it came to the norovirus genogroups, 24 cases (77.4%; 95% CI, 61.1%–93.8%) belonged to genogroup I, and genogroup II was not detected in isolation in any sample. Four samples tested positive for both genogroup I and II (12.9%; 95% CI, 3.6%–29.8%).
DiscussionAcute gastroenteritis is one of the leading causes of death in children aged less than 5 years,3 and the most frequent aetiology is viral. The percentage of cases of idiopathic AGE continues to be high, and can only be reduced by identifying the causative agent. In this study, we established the percentage of acute gastroenteritis cases in which norovirus could be identified as the causative agent thanks to the addition of the CerTest Norovirus GI+GII test to the microbiological workup of AGE.
The data showed an actual 4.1% increase in the second group in the percentage of samples with a microbiological diagnosis, which corresponded to 12 cases of norovirus detected in isolation.
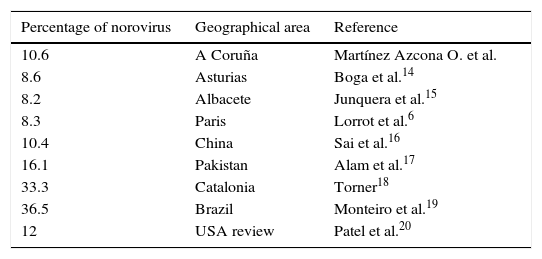
In Spain, rotavirus continues to be the leading cause of AGE.2,7 However, norovirus is growing in importance, and is now the second leading cause.2 Our data for norovirus were similar to those reported by other authors,6,12,13 as can be seen in Table 3. Only 2 authors found higher percentages of norovirus.18,19 Patel et al.20 made a systematic review of studies published before 2008, in which infections caused by norovirus amounted to 12% of severe cases, which is consistent with our findings.
Percentage of norovirus reported by different authors in different geographical areas.
| Percentage of norovirus | Geographical area | Reference |
|---|---|---|
| 10.6 | A Coruña | Martínez Azcona O. et al. |
| 8.6 | Asturias | Boga et al.14 |
| 8.2 | Albacete | Junquera et al.15 |
| 8.3 | Paris | Lorrot et al.6 |
| 10.4 | China | Sai et al.16 |
| 16.1 | Pakistan | Alam et al.17 |
| 33.3 | Catalonia | Torner18 |
| 36.5 | Brazil | Monteiro et al.19 |
| 12 | USA review | Patel et al.20 |
When it came to the distribution of norovirus genogroups, the most prevalent was GI, found in 77.4% of all positive samples. Strains of GII were not found in isolation, but there was a mixed infection in 12.9% of cases. These findings diverge from those published by Lorrot et al.,6 who observed a clear predominance of genogroup II, and especially the GGII.4 genotype, followed by genotype GGII.b. Buesa et al.21 declared GGII.4 the most prevalent genotype in sporadic cases of gastroenteritis in the paediatric age group. The results of Kirkwood et al.22 and Boga et al. followed a similar trend.14 Sai et al.16 also found that of the three detected genogroups—GII.3, GII.4 and GII.6—the most prevalent was GII.4. An article published by Hoehne and Schreier23 offers an overview of the genomic diversity of norovirus.
The prevalence of norovirus cases remained within a narrow range throughout the year. However, the relatively small number of cases that tested positive exclusively for norovirus (n=12) precluded a reliable statistical analysis. Lorrot et al.6 observed seasonal changes in the incidence of norovirus cases, with an initial peak in January and a smaller peak in September, coinciding with the peaks of rotavirus, which were much more marked. In contrast, Sai et al.16 reported an incidence peak between September and November for norovirus, while the peak for rotavirus shifted to the colder months of November through January.
In our study, we found that norovirus had an important role as a coinfecting agent, with a predominance of coinfection by norovirus and adenovirus (25.8% of cases) and by all 3 viruses under study (rotavirus, adenovirus and norovirus, another 25.8%). Coinfection with rotavirus and norovirus was detected in 9.7% of the samples. These data were consistent with previous findings,11 although the percentage was higher in our study (61.3%). In the reviewed literature, Li et al.24 described a positive correlation between rotavirus and adenovirus, on one hand, and norovirus GII and Salmonella, on the other.
To conclude, we observed an increase in the proportion of cases with an identified aetiological agent after the introduction of the CerTest Norovirus GI+GII diagnostic test.
Conflict of interestsThe authors have no conflict of interests to declare.
We thank María Teresa Seoane Pillado, statistics technician of the Unit of Clinical Epidemiology and Biostatistics of the Complejo Hospitalario Universitario de A Coruña; Amparo Otero Fernández, who provides administrative support for clinical trials at the Unit of Clinical Epidemiology and Biostatistics of A Coruña; and José Vázquez Tato, chemistry professor at the Universidad de Santiago de Compostela.
Please cite this article as: Martínez Azcona O, Vázquez Gómez L, Buyo Sánchez P, Díaz Soto R, Moldes Suárez LM. Gastroenteritis agudas y virus entéricos: impacto de la detección de norovirus. An Pediatr (Barc). 2017;87:143–147.