Monitoring of brain function using continuous electroencephalography (aEEG/cEEG) is an essential tool in the standard care of the term infant, and its use is growing in the premature infant as a biomarker of lesion and brain maturity. However, the placing of the electrodes is a great challenge, particularly in the extremely premature infant, which often discourages neuromonitoring. The aim of this study is to assess the different electrodes available, to select the one that best suits the peculiarities of the extremely premature infant, and evaluate its applicability in clinical practice.
Population and methodsWith the aim of designing a neuromonitoring study protocol using aEEG/cEEG in <28 weeks premature infants, an analysis was made of our experience with the type of electrodes available. The electrode that was considered most suitable for this population was chosen by assessing: the need of preparing the scalp, speed in positioning the electrodes, if the application was invasive or not, the possibility of repositioning, risk of skin injuries, sterility of the technique, and durability. The electrode chosen was used for continuous electroencephalographic monitoring started in the first 24 h of life, and maintained until at least 72 h of life.
ResultsThe electrodes evaluated were: subdermal needles, silver cups, and 2 types of self-adhesive electrodes (solid hydrogel and wet gel). The wet gel electrodes were chosen. They were used on 41 neonates with a mean gestational age of 25.8 ± 1.1 weeks. Good stable impedance was rapidly obtained, without the need of excessive manipulations, and no skin injuries were observed. The satisfaction of the staff involved in positioning them was very high.
ConclusionThe self-adhesive disposable electrodes with wet gel and integrated cable enabled the electrodes to be positioned rapidly and provided continuous non-invasive and good quality aEEG/cEEG monitoring in the extremely premature infant.
La neuromonitorización de la función cerebral mediante electroencefalografía continua (aEEG/cEEG) es una herramienta esencial en el cuidado estándar del niño a término, y de utilidad creciente en el niño prematuro como biomarcador de lesión y maduración cerebral. Sin embargo, la colocación de los electrodos supone un gran reto, especialmente en el niño prematuro extremo, desalentando frecuentemente su neuromonitorización. El objetivo de este estudio es analizar los diferentes electrodos disponibles, seleccionar el que mejor se adapta a las peculiaridades del niño prematuro extremo y evaluar su aplicabilidad en la práctica clínica.
Población y métodosCon motivo del diseño de un protocolo de estudio de neuromonitorización mediante aEEG/cEEG en niños prematuros <28 semanas, analizamos nuestra experiencia con los tipos de electrodos disponibles y seleccionamos el que consideramos más adecuado para esta población mediante la valoración de: necesidad de preparación del cuero cabelludo, rapidez de colocación, si se trataba de una aplicación invasiva, posibilidad de reposicionamiento, riesgo de lesiones cutáneas, esterilidad de la técnica y durabilidad. El electrodo elegido se utilizó para la monitorización continua electroencefalográfica iniciada en las primeras 24 h de vida y mantenida al menos hasta las 72 h.
ResultadosLos electrodos evaluados fueron: agujas subdérmicas, cucharillas de plata y 2 tipos de electrodos autoadhesivos (de hidrogel sólido y de gel conductor líquido). Los electrodos de gel conductor líquido fueron los elegidos. Se utilizaron en 41 neonatos con una edad gestacional media de 25,8 ± 1,1 semanas. Se obtuvo una buena impedancia duradera de forma rápida y sin necesidad de manipulaciones excesivas y no observamos lesiones cutáneas. La satisfacción del personal involucrado en su colocación fue muy elevada.
ConclusiónLos electrodos autoadhesivos desechables con gel conductor líquido y cable integrado permiten una colocación rápida y no invasiva para una monitorización aEEG/cEEG prolongada y de buena calidad en el niño prematuro extremo.
The applicability of continuous cerebral function monitoring by amplitude integrated electroencephalography (aEEG) in neonatal clinical practice is indisputable.1,2 The indications for its use in term neonates are well established.3–5 When it comes to preterm infants, recent studies have demonstrated its usefulness to detect acute brain injury, assess the maturation of cerebral electrical activity and to predict neurologic outcomes.6–20
The difficulty in correctly placing electrodes frequently delays initiation of monitoring, creates artefacts in the tracings that hinder their interpretation and/or results in multiple handlings of unstable patients. These hurdles are magnified in extremely preterm infants, especially in the first few days of life, discouraging neuromonitoring.21,22 The room available for manoeuvring is very limited, as these infants need to be in the incubator and frequently ventilatory support as well. The surface available to place the electrodes is also limited due to the small size of the head, and difficult to access since most of these infants require tight caps to hold nasal continuous positive airway pressure (nCPAP) devices. Time is another limitation, as loss of heat and humidity during prolonged handling could disrupt the thermal homeostasis of the infant and, in the most vulnerable patients, haemodynamic instability. Thus, despite it being a non-invasive technique, initiating aEEG monitoring requires considerable skill.23
The aim of our study was to analyse the different electrodes available for neurologic monitoring with aEEG or continuous electroencephalography (cEEG), select the type best suited to the particular characteristics of extremely preterm infants and assess its usefulness in clinical practice.
Patients and methodsWe conducted the study in a level IIIC neonatal unit equipped with cerebral function monitors since 2006. Different types of electrodes have been used from the time aEEG monitoring was introduced in the unit: subdermal needles (Ambu® Neuroline Subdermal Needle), silver cups (Pediatric Silver Cup Electrodes, Natus® Neurology), solid hydrogel self-adhesive electrodes (Neonatal Hydrogel Sensors, Natus® Neurology and Ambu® Neuroline 700) and wet gel self-adhesive electrodes, with a pre-attached lead wire (Ambu® Neuroline 720) and without (Ambu® BlueSensor N, used with Natus® Neurology reusable snap leads).
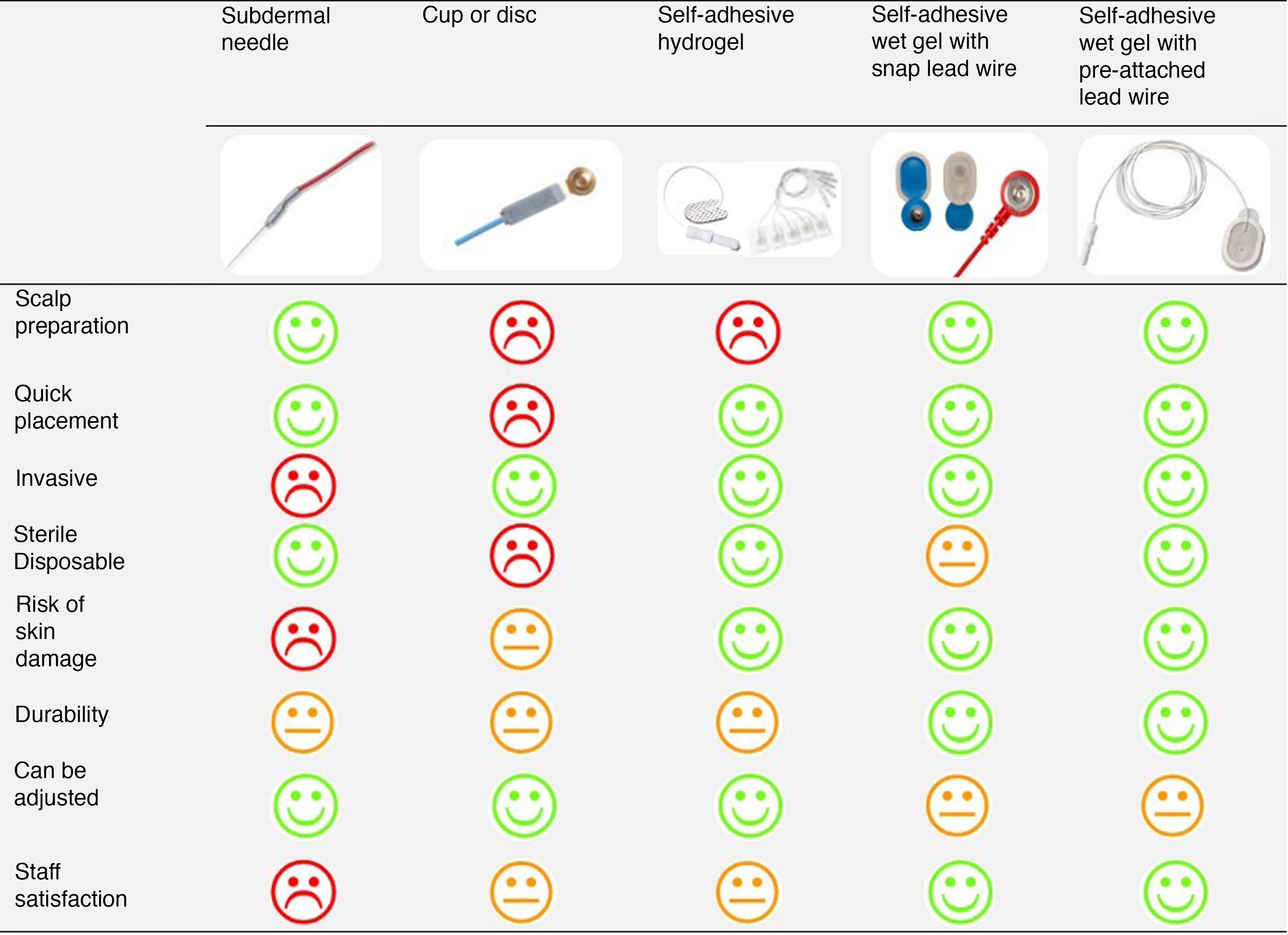
With the aim of designing a study protocol to analyse continuous neurologic monitoring in preterm infants delivered before 28 weeks’ gestation, we reviewed the experience of our unit with the use of the different types of electrodes available for aEEG/cEEG monitoring. Two neonatologists with expertise in aEEG monitoring carried out the assessment. The analysis was based in their own experience using the different electrodes in clinical practice and a survey of teaching nurses and nurses with more than 10 years’ experience in the unit. We assessed the need for prior preparation of the scalp to achieve an adequate impedance, the time required for electrode placement, whether the placement technique was invasive or not, whether it was possible to reposition the electrodes, the risk of cutaneous lesions in case of prolonged monitoring and the sterility and durability of electrodes.
The electrode that was eventually selected, a self-adhesive wet gel electrode with a pre-attached lead wire (Ambu® Neuroline 720), was used for cEEG monitoring in preterm infants delivered before 28 weeks’ gestation, initiated in the first 24 h post birth and maintained at least through 72 h. We used the modified International 10/20 montage model (10 electrodes/patient: Fp1, Fp2, C3, C4, T3, T4, O1, O2, ground and reference).23 The aEEG trend was derived from 3 channels (C3-T3, C4-T4, C3-C4).
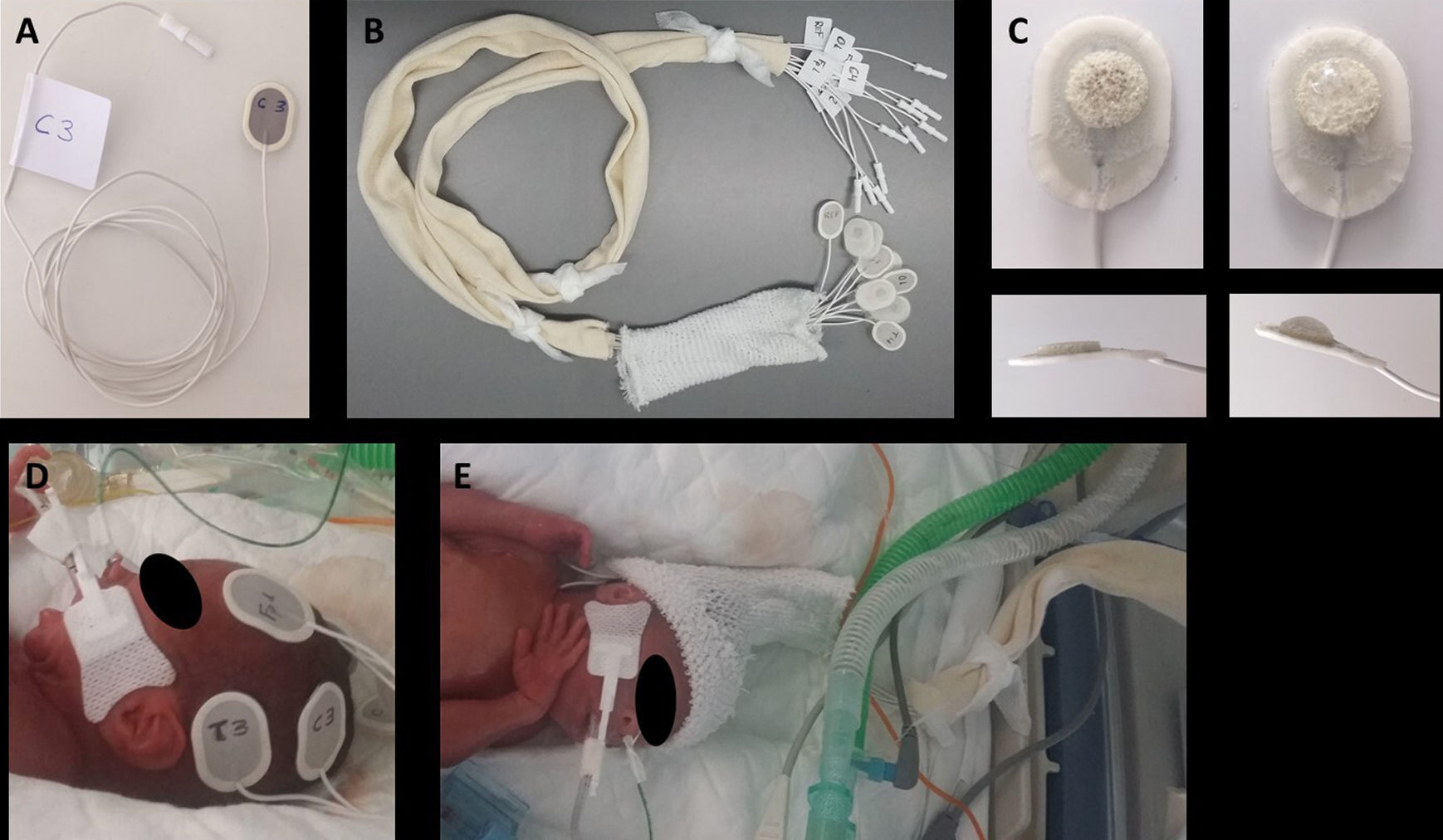
Electrode placement procedureTo minimise the time spent handling the patient and working inside the incubator and ensure uniform implementation of the procedure, we followed the steps proposed by Lloyd et al24 with some modifications: (1) label the electrodes at both ends with the corresponding anatomical sites: with marker in the back of the sensor end and with a label in the wire, close to the connector to the EEG amplifier, so that the electrodes can be connected to the headbox at a later time; (2) Group all the cables in a substantial length of tubular stockinette (Tubinet®) to prevent entanglement with other cables, infusion lines or tubing and reduce interference; (3) Group the electrodes of each hemisphere together to apply the electrodes to one side of the head and then the other, thus reducing head turning25 (Fig. 1).
Preparation and placement of electrodes: (A) Label both ends of each electrode with the corresponding anatomical site; (B) group all the cables in a tubular stockinette bandage; (C) apply a small amount of adhesive conductive gel to the sponge in the electrode; (D) apply all the electrodes for one side of the head, then all the electrodes for the other side; (E) Slip the tubular bandage over the head to serve as a holding cap.
The electrodes were introduced in the incubator a few minutes before placement to warm them up and facilitate adhesion. Then, the protective plastic lining was removed from the electrodes followed by application of an amount of conductive adhesive gel the size of a rice grain (Pâte ReegaPha Conductrice Tube, MEI). After placing each electrode in the corresponding site, pressure was exerted for a few seconds along its edges (as opposed to the area containing the sensor and gel) to achieve maximum adhesion. Patients that did not need a nCPAP cap were fitted with a cap made with a tubular net bandage (Tubifix®) to hold the electrodes. Monitoring was performed with the NicoletOne™ EEG System (Natus Medical Inc., USA). We also used a questionnaire regarding the parameters used in the initial selection of the optimal electrode, surveying the satisfaction of the nursing staff involved in the placement and maintenance of the electrodes used in the patients under study. Electrodes were removed with a cleaning solution (Brava® adhesive remover spray, Coloplast).
The study was approved by the Clinical Research Ethics Committee. We included patients in the study after obtaining signed informed consent.
We present quantitative data as mean ± standard deviation.
ResultsAnalysis of the pros and cons of the different types of electrodesAmbu® Neuroline Subdermal NeedleDoes not require previous preparation of the scalp save for the application of antiseptic and can be placed quickly. The drawback is that placement is invasive, breaking the skin barrier and thereby posing a risk of cutaneous lesions and infection. They allow prolonged monitoring, although they get displaced relatively frequently, requiring readjustment. They pose a risk of accidental needle stick injury in health care workers.
Pediatric Silver Cup Electrodes, Natus® NeurologyPlacement is non-invasive and can be repeated as many times as needed, and the electrodes are the smallest available (6 mm cup diameter). The drawback is that placement takes time, as the scalp must be prepared with an abrasive paste and each cup must be filled with conductive paste and taped to the scalp to achieve adequate fixation. They also get displaced often, are associated with a risk of skin lesions in lying positions and are not disposable.
Self-adhesive pre-gelled solid Hydrogel electrodes (Neonatal Hydrogel Sensors, Natus® Neurology and Ambu® Neuroline 700)These electrodes are flat and have a silver sensor that sits on a flexible layer of conductive and adhesive hydrogel that facilitates repositioning. Thus, the area of skin in contact with the electrode corresponds to the area of adhesion and measurement. The smallest electrodes in this category are manufactured by Ambu® (15 × 20 mm). Placement is quick and non-invasive, removal is easy, leaving no residue on the skin, and they are disposable. However, they require preparation of the scalp with an abrasive paste and, in our experience, they do not stay fixed long and frequently lose adequate contact with the scalp, requiring frequent reattachment.
Self-adhesive pre-gelled wet gel electrodes with pre-attached lead wire (Ambu® Neuroline 720) and without pre-attached wire (Ambu® BlueSensor N used with Natus® Neurology reusable snap leads)They have a silver sensor that sits on a small sponge soaked in wet conductive gel and surrounded by an adhesive acrylic tab (Fig. 2). Thus, in these electrodes, the measurement area is smaller than the area of contact with the skin and separate from the adhesion area. They do not require preparation of the scalp and can be placed quickly and non-invasively, the adhesion of the acrylate is strong and lasting, and the gel has a high conductivity. In prolonged recordings, the contact of the sensor with the scalp deteriorates as the conductive gel dries up, but this can be fixed without detaching the electrode by applying an adhesive conductive gel between the electrode tab and the scalp. However, if the electrode detaches completely from the scalp, it can no longer adhere to the skin and cannot be reused. Removal of the electrodes and, if used, any added adhesive conductive gel that has dried up, may require an adhesive removal spray.
The model with the pre-attached lead wire is flat (30 × 22 mm) and fully disposable. The model without the pre-attached lead wire is larger (44.8 × 22 mm) because it includes the connector for the wire, which, in turn, has the disadvantage of creating bulk where it attaches to the electrode and is not disposable.
Selection of the optimal electrodeFig. 3 presents the comparison of the different electrodes based on the parameters selected to determine their ease of use and appropriateness by 2 neonatologists experienced in EEG monitoring and the nurses of the neonatal intensive care unit.
Applicability to clinical practiceWe used self-adhesive wet gel electrodes with pre-attached lead wires (Ambu® Neuroline 720) in 41 infants born at a mean gestational age of 25.8 ± 1.1 weeks with a mean weight of 820 ± 186 g. The mean total time elapsed in preparation and electrode placement to initiation of monitoring was 30 min. An impedance of less than 10 kΩ in every electrode was achieved in the first in 36 patients (88%), with an impedance of less than 5 kΩ in every electrode in 25 patients (61%). The mean duration of aEEG/cEEG monitoring was 71 ± 17 h.
During monitoring, we made 375 measurements of impedance: the impedance was less than 10 kΩ in all electrodes in 286 tests (76%) and less than 5 kΩ in every electrode in 195 tests (52%). The electrodes did not have to be changed in any patient. The application of a small amount of adhesive conductive gel (Pate ReegaPha Conductrice Tube, MEI) under the self-adhesive disc with a plastic cannula connected to a syringe loaded with the gel sufficed to lower the impedance whenever the contact of a sensor with the scalp deteriorated. Cutaneous lesions were not detected in any patient. The staff involved in electrode placement and maintenance reported a high level of satisfaction. Electrodes were removed easily with the adhesive removal spray (Brava®, Coloplast).
DiscussionThis study outlines a systematic, simple, non-invasive and lasting method to place electrodes for continuous monitoring by aEEG/cEEG in extremely preterm infants. We tackled 2 challenges: (1) identifying the optimal electrodes for this subset of preterm infants, prioritising the minimization of invasiveness and handling both in the placement and the maintenance of the electrodes, and (2) being able to carry out prolonged monitoring while maintaining low impedance values and obtaining quality recordings.
The main foe we faced was impedance, and we prevailed. Impedance in this context is defined as the opposition to the passage of electric current between the surface of the cerebral cortex and the scalp, and when it comes to surface electrodes, it reflects the quality of the contact between the skin and the electrode. A high impedance in an electrode increases the likelihood that it will register other signals commonly found in the environment (respirators, infusion pumps, lights), creating artefacts in the recording. Subdermal needles achieve a low and stable impedance immediately without the need to exfoliate the skin.26 However, this method is not perceived positively by health care staff or families on account of its invasiveness. The American Clinical Neurophysiology Society27 consider its use contraindicated. The alternative to needles is the laborious preparation of the scalp with an abrasive paste or gel to use surface electrodes (self-adhesive and cup electrodes).24,28 This exfoliation removes the dry topmost layer of the epidermis and moistens the stratum corneum, thereby improving conductivity, but its use in extremely preterm newborns is discouraged due to the risk of cutaneous lesions. With self-adhesive wet gel electrodes, with the occasional application of a small amount of adhesive conductive gel, it is possible to achieve impedances below 5–10 kΩ without the use of the abrasive paste. While impedances below 5 kΩ are ideal, neurophysiology standards allow for impedances below 10 kΩ, especially in neonates, to avoid excessive handling and prevent cutaneous lesions.27 In infants delivered before 30 weeks’ gestation, the epidermis is excessively hydrated and constitutes a poor barrier that matures quickly after from 1 week post birth.29 This could explain why low impedances can be achieved without needing to exfoliate the skin in this population. However, wet gel hydrates the skin more and quicker than hydrogel, which also contributes to improving conductivity, and the gel used in wet gel electrodes is highly conductive. Thus, the use of self-adhesive wet gel electrodes can decrease handling times and the potential discomfort caused by the preparation of the scalp, in addition to achieving lower impedances and improving the quality of the signal.
We prepared the electrodes following a process similar to the one described by Lloyd et al.,24 which allowed reducing preparation time in the incubator and the number of times the infant’s head needed to be moved. It is also very useful to have the electrodes labelled on the end of the cable near the EEG amplifier, as it allows quick disconnections and reconnections in case, for instance, a cap needs to be placed on the infant due to accidental extubation for urgent switching to non-invasive ventilation with CPAP. Lloyd et al.24 used solid hydrogel electrodes successfully. In our experience, hydrogel electrodes require frequent repositioning, although we did not secure them with tape and conductive paste, as the authors described. In addition, to maintain the electrodes in place and have easy access to them, the authors used nCPAP caps cut along the anterior midline (in patients with and without non-invasive ventilation) that they then closed with the velcro that was part of the cap and adhesive tape. They reported that this did not prevent the tight seal required for nCPAP, but we do not think this is an adequate solution given the importance of the fit of the cap to keep the interface in the correct position. Schumacher et al.30 also reported good results with the use of solid hydrogel electrodes using a holding cap similar to the one described by Lloyd et al.,24 but specifically designed for the purpose. The adhesiveness of wet gel electrodes is greater compared to hydrogel electrodes, as the former use acrylate as an adhesive, so they do not need an additional system to fix the electrodes, such as adhesive tape, although nCPAP caps or caps fashioned with tubular stockinette can also hold them in place by keeping the cables organized and preventing their displacement during handling. On the other hand, even if they are not accepted in the minimum technical standards,27 both methods24,30 have been found to tolerate higher impedances (20 and 40 kΩ, respectively) based on the results of Ferree et al.,31 who compared recordings obtained with impedances of 10 vs 40 kΩ and found no significant differences.
Hydrogel electrodes and the adhesive tape used to hold cups and needles do not adhere well when there is hair on the scalp, sometimes requiring shaving the area. In such cases, wet gel electrodes may also require preparation of the scalp to achieve an adequate impedance, but the presence of hair does not diminish their ability to adhere.
The acrylic adhesive of the wet gel electrodes is therefore a great advantage when it comes to their placement, but at the same time it is a drawback when it comes to their removal. An adhesive removal spray is required to remove them without causing discomfort. Once removed, the electrodes can no longer adhere to the skin and therefore, unlike the other types of electrodes, cannot be reattached. However, also unlike the rest, when the sensor is no longer making adequate contact with the scalp, the problem can be resolved without removing the electrode to prepare the skin anew, as adding a small amount of conductive gel under the adhesive disc is enough to restore contact. We ought to emphasise the importance of applying very small amounts of gel under the sensors, as excessive amounts may create electrical bridges between electrodes or create a crust upon drying that would act as a barrier and increase the impedance, which would require changing the electrodes.
Whether the electrode has or not a pre-attached lead wire is a factor worth considering in preterm infants, but is not considered important in our unit when it comes to term infants. Due to the very small size of the head of preterm infants, the additional space required by the press studs may interfere with the placement of CPAP caps, add points of pressure on the skin frequently result in a poor fit of the stud and connector joint against the scalp, which would instead be hanging and pulling on the electrode.
Although our study used conventional EEG monitoring, aEEG monitoring, that is, monitoring with fewer electrodes, is currently being used in clinical practice. The system used in the study requires 3–6 electrodes per patient. Nevertheless, the time needed to initiate monitoring (preparation, opening incubator doors and handling patient) would be shorter in everyday clinical practice in neonatal units.
All the staff members involved in the placement and maintenance of the electrodes were clearly satisfied with them. Many openly admitted that using this system and these electrodes they did not postpone or avoid initiation of neurologic monitoring, and what used to be perceived as a tedious challenge is now approached with a proactive attitude in our unit.
The main limitation of this study is that we did not carry out a prospective and direct comparison of the different types of electrodes. To date, El Ters et al32 have been the only authors to publish results of a prospective comparative study of electrodes. They compared hydrogel and cup electrodes and found no difference in safety or the quality of the recordings. In our experience, self-adhesive wet gel electrodes offer higher conductivity and better adhesion compared to solid hydrogel electrodes, allowing for longer monitoring and producing recordings of higher quality. To date, there are no studies in the literature analysing the use of self-adhesive wet gel electrodes in the neonatal population.
In future, it would be useful to carry out a prospective study to compare solid hydrogel and self-adhesive wet gel electrodes. In any case, the future lies in dry sensor electrodes33 integrated in caps designed to also be able to hold CPAP devices, although there is still a long way to go.
ConclusionSelf-adhesive wet gel electrodes with pre-attached lead wires allow rapid and non-invasive electrode placement for prolonged, high-quality aEEG/cEEG monitoring in extremely preterm infants. Overcoming the challenges of electrode placement will facilitate neuromonitoring in this population and improve the quality of care in neonatal intensive care units, given the growing evidence that corroborates the clinical benefits of cerebral function monitoring by aEEG in preterm infants.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank all nurses and nursing assistants in the neonatal unit for their collaboration and substantial contribution to this study.
Please cite this article as: Cordeiro M, Peinado H, Montes MT, Valverde E. Evaluación de la idoneidad y aplicabilidad clínica de diferentes electrodos para la monitorización aEEG/cEEG en el niño prematuro extremo. An Pediatr (Barc). 2021;95:423–430.