The resistance to antibiotics of Helicobacter pylori (H. pylori) is the main factor that affects current therapeutic treatments. The main objective of this study is to describe the pattern of antibiotic resistances in children with an infection due to H. pylori.
Patients and methodsAn observational, retrospective study was conducted from 2014 to 2019, which included patients between 5 and 17 years old, on whom a gastroscopy, with a gastric biopsy culture positive for H. pylori, and an antibiotic sensitivity study was performed. The antibiotic sensitivity studies were performed using an epsilometer (E-test). The cut-off points to define the resistances were those proposed by the European Committee on Antimicrobial Susceptibility Testing — EUCAST. The eradication study was performed using the 13C-urea breath test or the H. pylori monoclonal test in faeces 6–8 weeks after finalising the treatment.
ResultsThe study included 80 patients (63.8% females), with a mean age of 11.9 years (SD ± 2.7 DS). Over one-third (38.8%) of the patients had received previous treatment for H. pylori. In the endoscopy, peptic ulcer lesions were observed in 10% of patients. More than two-thirds (67.5%) had resistance to at least one drug. 16.3% presented double resistance. The primary resistances were: clarithromycin, 44.9%, metronidazole 16.3%, levofloxacine 7.9%, and amoxicillin 2%. Patients that received treatment according to the new ESPGHAN 2017 guidelines had significantly higher eradication rates compared to those that received treatment according to previous guidelines (80% vs. 55.8%, P = 0.04).
ConclusionsThe high rate of H. pylori resistances, and as a result, the low eradication rates, are still a very important cause for concern. The first line treatment, when this is indicated must be given following the antibiotic sensitivity studies, and in the cases where these cannot be done or are not available, at least in accordance with the regional resistance rates. The correct application of the new guidelines significantly improves the eradication rate.
Las resistencias antibióticas de Helicobacter pylori (H. pylori) son el principal factor que afecta a la eficacia de los regímenes terapéuticos actuales. El objetivo principal del estudio es describir el patrón de resistencias antibióticas en ni˜nos con infección por H. pylori.
Pacientes y métodosEstudio observacional retrospectivo de 2014 a 2019 en el que se incluyen pacientes entre 5–17 años a los que se realizó gastroscopia, con cultivo de biopsia gástricapositivo para H. pylori y estudio de sensibilidad a antibióticos. Los estudios de sensibilidad antibiótica se realizaron mediante E-test. Los puntos de corte para definir las resistencias fueron los propuestos por el EUCAST. El estudio de erradicación se realizó con test del aliento con urea marcada con C 13 o test monoclonal de antígeno de H. pylori en heces a las 6–8 semanas de finalizar el tratamiento.
ResultadosOchenta pacientes (63,8% mujeres). Media de edad 11,9 años (±2,7 DS). Un 38,8% habían recibido tratamiento previo para H. pylori. Un 10% presentaron en la endoscopia lesiones ulcerosas pépticas. El 67,5% presentaba resistencia al menos a un fármaco. Un 16,3% presentaron doble resistencia. Las resistencias primarias fueron: claritromicina 44,9%, metronidazol 16,3%, levofloxacino 7,9% y amoxicilina 2%. Los pacientes que recibieron tratamiento acorde a las nuevas guías ESPGHAN 2017 presentaron tasas de erradicación significativamente superiores en comparación con los que recibieron tratamiento acorde a las guías previas (80% vs. 55,8% p = 0,04).
ConclusionesLa alta tasa de resistencias de H. pylori y, en consecuencia, las bajas tasas de erradicación, siguen siendo una preocupación muy importante. El tratamiento de primera línea, cuando esté indicado debe hacerse guiado por estudios de sensibilidad antibiótica y en los casos en el que no se puedan realizar o no estén disponibles, al menos de acuerdo con las tasas regionales de resistencia. La aplicación correcta de las nuevas guías mejora de forma significativa el nivel de erradicación.
It is estimated that infection with Helicobacter pylori (H. pylori) affects approximately 50% of the global population and plays a key role in the development of several gastrointestinal diseases, such as chronic gastritis, peptic ulcer and stomach cancer.1 The infection is usually acquired in childhood, but, compared to adults, children are unlikely to develop complications. Furthermore, recurrence after successful eradication may be fairly frequent in children (recurrence within 1 year of up to 20% in children under 10 years).2
Recommendations for diagnosis and treatment in children and adolescents differ from adult guidelines because the risk-benefit ratio changes based on age.3 In children, in absence of peptic ulcer disease, defined as development of gastric or duodenal sores and ulcers, eradication of H. pylori is not associated with symptom improvement.4,5 The main objective in children with gastrointestinal symptoms should be to assess for potential underlying causes and not just colonization by H. pylori.6
Contrary to the management in adults, the “test and treat” strategy (non-invasive diagnostic testing and treatment) is not indicated in paediatric patients. Invasive diagnosis by means of an endoscopic examination is only indicated in patients with suspected peptic ulcer disease. Treatment is indicated in patients with confirmed peptic ulcer disuse associated with H. pylori infection. In patients with gastritis associated with H. pylori in absence of peptic ulcer disease, the decision whether to use eradication therapy will be made on a case-by-case basis in agreement with the patient and the family.7
Antibiotic resistance is the main factor that affects the efficacy of current eradication regimens. The prevalence of drug resistance varies based on the geographical area, patient age, previous administration of eradication regimens and antibiotic use in the general population.8,9 Since antibiotic resistance is an ever-changing phenomenon that differs between geographical areas, it is important to carry out epidemiological surveillance studies to obtain updated prevalence data to help clinicians select the best possible treatment.
The primary objective of our study was to describe the pattern of antibiotic resistance in paediatric cases of infection by H. pylori. Our secondary objectives were to describe the rate of eradication in patients treated based on results of antimicrobial susceptibility testing in adherence with the current recommendations of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) at each given time, and to identify risk factors associated with failed eradication.
Sample and methodsWe conducted a retrospective observational study including all patients aged 5–17 years given a diagnosis of H. pylori infection between January 2014 and December 2019 that underwent a gastroscopy and culture of a gastric biopsy with isolation of H. pylori and antibiotic susceptibility testing, whether or not they underwent treatment or testing for assessment of eradication.
Samples were cultured in medium selective for H. pylori (PYL, BioMerieux, France). Antibiotic susceptibility testing was performed with Etest strips (BioMerieux, France). We established antibiotic susceptibility based on the minimum inhibitory concentration (MIC) values proposed as cut-off points in 2011 by the European Committee on Antimicrobial Susceptibility Testing (EUCAST): amoxicillin, >0.12 mg/L; clarithromycin, >0.5 mg/L; metronidazole, >8 mg/L; levofloxacin, >1 mg/L; tetracycline, >1 mg/L; rifampicin, >1 mg/L.10
In patients who received it, treatment was selected based on the antibiotic results and in adherence with current ESPGHAN guidelines.
The assessment of eradication was performed with a 13C urea breath test (UBTest Otsuka Pharma) or a monoclonal antibody-based stool antigen test (H. pylori Turbidimetría, Materlab) 6–8 weeks after completion of treatment.
We collected demographic data, such as age, sex and country of birth, and clinical data, including symptoms, comorbidities, previous treatment, endoscopic findings, drug resistance profiles and eradication rates. We considered drug resistance primary if patients had not undergone eradication therapy in the past, and secondary when they had undergone treatment without success before performance of the culture. We defined double resistance as resistance to clarithromycin and metronidazole.
We conducted a descriptive analysis, summarising qualitative variables as frequency and percentage distributions and quantitative variables as mean and standard deviation or median and interquartile range. The primary endpoint was the frequency of drug resistance calculated with the corresponding 95% Wilson interval. We performed an exploratory univariate analysis to identify factors associated with the presence of drug resistance and with eradication. We compared qualitative variables by means of the chi square test or Fisher exact test and qualitative variables by means of the Student t test for independent samples or the nonparametric Mann-Whitney U test, depending on the shape of the distribution. All tests were bilateral, and statistical significance was defined as a p-value of less than 0.05.
Ethical considerations: the study protocol was reviewed and approved by the Ethics Committee on Clinical Research with Drugs (CEIm).
ResultsThe study included 80 patients (2014: n = 12; 2015: n = 15; 2016: n = 15; 2017: n = 9; 2018: n = 15; 2019: n = 14), 63.8% of who were female. The mean age at diagnosis was 11.9 years (SD, ±2.7). In 82.5% of cases, the country of birth was Spain, while 7.5% of patients were from Eastern Europe, 3.7% from South America, 3.7% from North Africa and 2.5% from Asia. The most frequent presenting complaint was epigastric pain (57.5%), followed by pain in other abdominal regions (22.5%), nausea/vomiting (5%) and refractory iron-deficiency anaemia (2.5%). In 12.5% of patients, the infection manifested with other symptoms or was a chance finding after performance of an endoscopic examination for other reasons. The most frequent gastrointestinal comorbidities were gastro-oesophageal reflux (13.7%), coeliac disease (7.5%) and eosinophilic oesophagitis (5%). In 10% of patients, the endoscopic examination found peptic ulcers described as gastric or duodenal erosions or ulcers. There was a history of previous failed H. pylori therapy in 38.8% of the patients. Seventy-four of the patients (92.5%) received eradication therapy after the endoscopy and culture, which varied based on the results of antibiotic susceptibility testing. All patients that received treatment underwent testing to assess the success of eradication 6–8 weeks after completion of the regimen.
Overall, during the study period, 67.5% of patients (n = 54; 95% confidence interval [CI], 56.6%–76.8%) had strains that were resistant to at least one drug: clarithromycin in 52.5% (n = 42; 95% CI, 41.7%–63.1%), metronidazole in 31.3% (n = 25; 95% CI, 22.2%–42.1%), levofloxacin in 7.9% (n = 5/63; 95% CI, 3.4%–17.3%), amoxicillin in 7.5% (n = 6; 95% CI, 3.5%–15.4%). Double resistance to clarithromycin and metronidazole was found in 16.3% (n = 13; 95% CI, 9.8%–25.8%).
Patients that had not received eradication therapy in the past were less likely to be infected by resistant strains compared to those that had undergone treatment unsuccessfully (primary resistance, 57.1% vs secondary resistance, 83.9%; P = 0.01) (Table 1). A greater proportion of patients born in Spain had strains resistant to at least one drug and there was also a significantly greater proportion of these patients with clarithromycin-resistant strains compared to patients born outside of Spain, none of whom had strains resistant to this antibiotic (63.6% vs 0%; P = 0.001). When it came to all other antibiotics, we did not find significant differences in the prevalence of drug resistance based on patient sex, age or country of birth (Table 2).
Prevalence of antimicrobial resistance.
| Overall resistance (%) | Primary resistance (%) | Secondary resistance (%) | P | ||||
|---|---|---|---|---|---|---|---|
| n = 80 | n = 49 | n = 31 | |||||
| Amoxicillin | 6 | 7.5% | 1 | 2.0% | 5 | 16.1% | 0.03 |
| Clarithromycin | 42 | 52.5% | 22 | 44.9% | 20 | 64.5% | 0.087 |
| Metronidazole | 25 | 31.3% | 8 | 16.3% | 17 | 54.8% | <0.001 |
| Double resistance | 13 | 16.3% | 2 | 4.1% | 11 | 35.5% | <0.002 |
| Levofloxacin (n = 63) | 5 | 7.9% | 3 | 7.9% | 2 | 8.0% | 1 |
| Tetracycline | 0 | 0 | 0 | – | |||
| Rifampicin (n = 38) | 0 | 0 | 0 | – | |||
Risk factors for development of resistance.
| Sensitive | Resistant | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Overall resistance (to any antimicrobial) | |||||
| Age | 11.7 ± 2.6 | 12 ± 2.7 | 0.683 | ||
| Sex | |||||
| Female | 16 | 31.4% | 35 | 68.6% | 0.775 |
| Male | 10 | 34.5% | 19 | 65.5% | |
| Born in Spain | |||||
| Yes | 17 | 25.8% | 49 | 74.2% | 0.01 |
| No | 9 | 64.3% | 5 | 35.7% | |
| Previous treatment | |||||
| Yes | 5 | 16.1% | 26 | 83.9% | 0.015 |
| No | 21 | 42.9% | 28 | 57.1% | |
| [0.1-6] | |||||
| Clarithromycin | |||||
| Age | 11.4 ± 2.7 | 12.4 ± 2.6 | 0.112 | ||
| Sex | |||||
| Female | 21 | 41.2% | 30 | 58.8% | 0.133 |
| Male | 17 | 58.6% | 12 | 41.4% | |
| Born in Spain | |||||
| Yes | 24 | 36.4% | 42 | 63.6% | <0.001 |
| No | 14 | 100.0% | |||
| Previous treatment | |||||
| Yes | 11 | 35.5% | 20 | 64.5% | 0.11 |
| No | 27 | 55.1% | 22 | 44.9% | |
| [0.1-6] | |||||
| Metronidazole | |||||
| Age | 12.2 ± 2.5 | 11.3 ± 2.9 | 0.188 | ||
| Sex | |||||
| Female | 35 | 68.6% | 16 | 31.4% | 0.975 |
| Male | 20 | 69.0% | 9 | 31.0% | |
| Born in Spain | |||||
| Yes | 46 | 69.7% | 20 | 30.3% | 0.755 |
| No | 9 | 64.3% | 5 | 35.7% | |
| Previous treatment | |||||
| Yes | 14 | 45.2% | 17 | 54.8% | <0.001 |
| No | 41 | 83.7% | 8 | 16.3% | |
| Double resistance | |||||
| Age | 11.9 ± 2.6 | 11.9 ± 3.1 | 0.969 | ||
| Sex | |||||
| Female | 40 | 78.4% | 11 | 21.6% | 0.119 |
| Male | 27 | 93.1% | 2 | 6.9% | |
| Born in Spain | |||||
| Yes | 53 | 80.3% | 13 | 19.7% | 0.11 |
| No | 14 | 100.0% | |||
| Previous treatment | |||||
| Yes | 20 | 64.5% | 11 | 35.5% | <0.001 |
| No | 47 | 95.9% | 2 | 4.1% | |
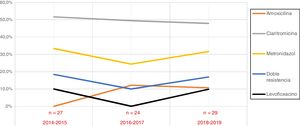
When we analysed the temporal trends in drug resistance throughout the study period, we did not find any significant changes during these years (Fig. 1).
All patients that were treated received treatment guided by the results of antimicrobial susceptibility testing and in adherence with the current ESPGHAN recommendations. The overall success rate of eradication therapy was 65.8%, with a higher proportion of successful eradication in patients that had not been treated previously (71.1% vs 57.1%; P = 0.31). Furthermore, the rate of eradication was lower in patients infected by double-resistant strains compared to the rest of patients (50% vs. 68.9%; P = 0.0.31). In patients treated in adherence with the new ESPGHAN guidelines from 2017, the eradication success rate was significantly higher compared to patients treated according to earlier recommendations (80% vs 55.8%; P = 0.04). The eradication success rate in patients treated in the last year under study (2019) was 100% (n = 12/12).
DiscussionWe found a very high prevalence of antibiotic resistance in our region during the period under study, comparable to the prevalence previously described in Spain.11–14 The prevalence of resistance to different drugs remained stable in the period under study and were similar to the figures reported in other countries in Southern Europe, where resistance to clarithromycin is noticeably more frequent compared to the rest of Europe.15–18
As for the risk factors for the presence of drug resistance, we found that prior failure of eradication therapy was a clear predictor of resistance. Therefore, in cases in which treatment is indicated, it is essential to prescribe treatment based on antibiotic susceptibility test results to prevent treatment failure and the development of secondary drug resistance.
Primary resistance to clarithromycin is markedly more prevalent in children (44.9%) compared to the prevalence described in adults in our region (14.7%–22%).19–23 The higher prevalence of primary resistance in children compared to adults suggests in vivo drug resistance development during childhood. The alarming frequency of clarithromycin resistance in patients that had not undergone eradication therapy in the past may be explained by the growing use of macrolides commonly used for empirical treatment of H. pylori and other common infections of childhood, chiefly of the respiratory tract. Clarithromycin should never be used as first-line treatment if culture or antimicrobial susceptibility testing has not been performed or H. pylori failed to grow in culture, as the prevalence of resistance far exceeds 20%.
On the other hand, primary resistance to metronidazole and levofloxacin are clearly less prevalent compared to the prevalence found in studies conducted in adults in our region (16.3% vs 27%–40% and 7.9% vs 13.9%–38%, respectively).20–23 Although quinolones have not been authorised for use in the paediatric population, the prevalence of primary resistance to levofloxacin in children is considerable (7.9%), which may be due to resistance in the causative strain and transmission of infection within the household from adults to children. Children most frequently acquire H. pylori from an infected parent, usually the mother.24
The prevalence of peptic ulcer disease in our study was 10%. This was consistent with the findings of a European multicentre study of drug resistance, which found evidence of ulcerative lesions in 6.8% of patients and 10.4% of those aged more than 11 years.15
The new ESPGHAN guidelines for the management of infection by H. pylori in children and adolescents were published in June 2017.3 These guidelines underscore that the main goal of the medical evaluation of children with gastrointestinal symptoms should be to determine the underlying cause of the symptoms, and not just to assess for the presence of H. pylori infection. They discourage testing for detection of H. pylori in children with functional abdominal pain, as well as “test and treat” approaches (non-invasive diagnosis and treatment), contrary to management guidelines for adults. The recommended approach to diagnosis of infection by H. pylori in children is through invasive testing, with performance of an endoscopic examination, gastric biopsy and culture. Eradication treatment, when indicated, should be prescribed based on the results of antimicrobial susceptibility testing. The new guidelines proposed significant changes to pharmacological treatment compared to previous guidelines, including longer duration of treatment and higher doses of proton pump inhibitors (PPIs), especially in young children (dose of 1.5–2.5 mg/kg/day).
When we analysed whether these new treatment recommendations resulted in improved eradication success rates, we found a significant increase in successful eradication in patients treated in adherence with the updated ESPGHAN guidelines.
To avoid development of secondary drug resistance in H. pylori strains involved in infections, the eradication success rate has to exceed 90% in the per-protocol analysis.25 However, this target is rarely met in most recent paediatric trials. In our study, while we observed a significant improvement in eradication rates in recent years, they were still below the desired target. Several factors may play a key role in this outcome, such as the high prevalence of antimicrobial resistance and the sizeable proportion of patients in our region infected by double-resistant strains, a subset in which the rate of successful eradication is lower.
The growing number of paediatric patients infected with multidrug-resistant strains warrants a revision of current treatment regimens. Second-line antibiotics, such as quinolones or tetracycline, are not authorised for use in the paediatric population or only authorised starting at a certain age. Rifabutin has been used successfully in adults, but may cause drug resistance problems in the treatment of tuberculosis, so treatment options in these patients are very limited. Since in vitro resistance to metronidazole can be overcome in vivo using very high doses and prolonged courses of treatment, a European multicentre study analysed the frequency of successful eradication and the side effects of a 2-week course of high-dose triple therapy (amoxicillin at ∼75 mg/kg/day, metronidazole at ∼25 mg/kg/day and esomeprazole at ∼1.5 mg/kg/day) in paediatric patients with infection by double-resistant strains confirmed by culture. The study found a rate of successful eradication of 66% in the intention-to-treat analysis (95% CI, 54%–78%) and of 73% in the per-protocol analysis (95% CI, 60%–86%).26 Although these rates are better compared to previous studies, they are still far from the established target. It must be taken into account that this study was conducted before the publication of the current guidelines and used lower PPI doses (∼1.5 mg/kg/day) than currently recommended e (1.5–2.5 mg/kg/day). The current guidelines recommend treatment of infection by double-resistant strains with a 14-day course of high-dose triple therapy (PPI-amoxicillin-metronidazole) or quadruple therapy with bismuth (age < 8 years: bismuth-PPI-amoxicillin-metronidazole; age > 8 years: bismuth-PPI-metronidazole-tetracycline).
Besides antibiotic resistance in H. pylori, there are other possible reasons for treatment failure, such as lack of adherence, inadequate dosage or duration of treatment, rapid metabolising of certain PPIs, bacterial virulence factors, ineffective penetration of antibiotics in the gastric mucosa and inactivation of antibiotics at low gastric pH.
A recent study analysed the role of adherence in treatment failure in paediatric patients that received treatment tailored to antimicrobial susceptibility.27 The study found that eradication in children with a concordance between prescribed and ingested drugs above 90% was successful in 89.9% of the patients, compared to 36.8% of the patients who did not adhere to treatment. Therefore, it is essential that patients and their families are informed of the importance of adequate adherence to treatment, as it can have a significant impact in improving the rate of successful eradication.
The high prevalence of antimicrobial resistance in H. pylori and, therefore, the low frequency of successful eradication, continue to be a significant concern. Therefore, prescription of first-line treatment should be tailored to antimicrobial susceptibility and, in cases in which susceptibility testing is not available or cannot be performed, based on the known regional prevalence of antimicrobial resistance. Correct implementation of the most recent guidelines significantly improves the rate of successful eradication.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Botija G, García Rodríguez C, Recio Linares A, Campelo Gutiérrez C, Pérez-Fernández E, Barrio Merino A. Resistencias antibióticas y tasas de erradicación en infección por Helicobacter pylori. An Pediatr (Barc). 2021;95:431–437.
Previous presentation: preliminary results of the study were presented as a brief oral communication at the congresses of the Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica (SEGHNP) held in Gijon, Spain, in 2016 and in Santander, Spain, in 2019.