The informed consent of the minor is a fundamental requirement of paediatric research. There is a lack of harmonisation as regards the age of the mature minor to consent, and there are no systematic tools available to assess competence in decision-making capacity. The objective of this work is to analyse the ethical and legal situation of consent by minors, as well as studies that use an objective assessment tool in the mature minor.
Material and methodsSystematic review of scientific articles in PubMed, Embase and the Grey Literature, published with keywords “informed consent minors”, without date restriction until March 2019. Abstracts and a selection of complete articles were reviewed following a protocol including identification, screening, eligibility, and inclusion.
ResultsOf the 260 records identified, 139 were excluded. After categorising the resulting 121 publications, 13 were finally selected following the eligibility criteria, including 7 articles on international ethical and legal regulations and 6 on understanding and decision- making capacity assessment. The MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR) semi-structured interview was used in 4 studies, including different age ranges (6–21 years) in healthy and sick children.
ConclusionsThe semi-structured MacArthur interview adapted to adolescents could be an appropriate tool with robust psychometric measures for assessing competence for the informed consent of minors between 9 and 12 years of age. The regulation of informed consent in paediatric research should consider this evidence.
El consentimiento informado del menor es un requerimiento fundamental de la investigación pediátrica. Actualmente existe una desarmonización en cuanto a la edad del menor maduro para consentir y no se dispone de herramientas sistemáticas para evaluar la competencia en la capacidad de decisión. El objetivo de este trabajo es analizar la situación ética y legal del consentimiento en menores, así como los estudios que utilizan un instrumento objetivo de evaluación en el menor maduro.
Material y métodosRevisión bibliográfica de artículos científicos en PubMed, Embase y Literatura gris, publicados con palabras clave «informed consent minors», sin restricción de fecha hasta marzo 2019. Se revisaron los abstracts y una selección de los artículos completos siguiendo un protocolo de identificación, cribado, elegibilidad e inclusión.
ResultadosDe los 260 registros identificados, se excluyeron 139. Tras categorizar los 121 artículos resultantes, finalmente se seleccionaron 13 siguiendo los criterios de elegibilidad, incluyéndose 7 artículos sobre normativa ética y legal internacional, y 6 sobre evaluación de comprensión y capacidad de decisión. En 4 estudios se ha utilizado la entrevista semiestructurada McCarthur Competency Assessment Tool for Clinical Research (MacCAT-CR), en diferentes rangos de edad (6–21 años), niños sanos y con alguna patología.
ConclusionesLa entrevista semiestructurada McCarthur adaptada a adolescentes podría ser una herramienta adecuada con medidas psicométricas robustas para la valoración de competencia para el consentimiento informado de menores entre 9 y 12 años. La regulación del consentimiento informado en investigación pediátrica debería ser receptiva a estas evidencias.
Clinical research in paediatric subjects has grown significantly in recent decades, and public health authorities recognise the need of performing more trials in this vulnerable subset of the population that is underrepresented in clinical trials.1,2 Off-label drug prescription is frequent in the paediatric population, for instance of growth hormone replacement therapy and other drugs used in paediatric endocrinology practice.3,4
Children have particular physiological, psychological and developmental characteristics that require performance of specific clinical trials of new drugs and vaccines to determine their pharmacodynamics, pharmacokinetics and safety profile and improve treatment options in this age group.5 Paediatric clinical research has unique and complex ethical implications. Due to the vulnerability of minors and their lack of a legal right to consent, measures must be taken to protect their rights and to minimise the risks that they are exposed to.
Minor consent or assent is an essential requirement for research in paediatrics and clinical decision-making that is contemplated in international codes of ethics (2013 Declaration of Helsinki, Council for International Organizations of Medical Sciences, ICH-GCPs International Conference Harmonization-Good Clinical Practices, 2015 Nuffield council on Bioethics) and current law at the state level.
Consent and assent are not the same. Consent represents a formal decision to allow participation in research provided by an adequately informed individual that has the legal ability to agree to such participation. Assent involves a similar agreement but by an individual that does not have the legal capacity to consent and who requires the consent of a legally authorised individual (in the case of a minor, the parents or legal guardians). In the words of the Declaration of Helsinki, “when a potential research subject who is deemed incapable of giving informed consent is able to give assent to decisions about participation in research, the physician must seek that assent in addition to the consent of the legally authorised representative.”
The consent/assent process in a minor involves significant challenges for researchers, among them the comprehension of the written information provided to the patient. Usually, written information is not adapted to the reading level of potential participants (adults and minors alike), which hinders comprehension and therefore the ability to consent.6–8
When it comes to minors, regardless of the legal age of consent (for, when they are not of age, assent should still be obtained if they are able to provide it), it is essential to provide information fitting their comprehension level through the correct choice of language, format and volume of information, in addition to verifying their understanding of the contents.9 It is important to consider differences in their decision-making capacity based not only on age but also on their level of development, social, environmental and family-related factors and their medical condition.10
Generally, children start developing the ability to understand research procedures and how they may differ from routine medical care from age 7 years, so they could start to agree to participation at this age; some authors propose personalising this process, adapting it to each child.11–13
At present, no standardised tool is available to assess the ability of minors to make decisions and there is a lack of consensus on the age of consent to either medical research or medical care, and the determination of the maturity, understanding and competence of the minor rests on judgment of the researcher or clinician.10
The aim of our study was to analyse the following aspects through a literature review: 1) the current ethical guidelines and law on informed consent (IC) in minors regarding medical care decisions and/or participation in clinical research and 2) studies that use objective tools to assess the competence of minors for IC in decisions related to medical care and research protocols.
Material and methodsStudy design and inclusion criteria: a single reviewer analysed scientific articles and grey literature according to the following protocol:
Identification stageSearch in the Medline and EMBASE databases using the key words “Informed consent, minors, informed consent by minors” for articles in English or Spanish published before March 2019 with no recency restrictions. Search for grey literature on this topic in Google scholar and of published reports of the H2020 i-consent project (Improving the guidelines of informed consent, including vulnerable populations, under a gender perspective).
Screening stageThe identified sources were screened applying the following criteria:
Inclusion criteria: articles with titles, key words or abstracts including terms such as: competence evaluation, comprehension, decision-making capacity and tools used for IC, legal and ethical standards, cut-off age for minor assent, IC or assent form content and readability.
Exclusion criteria: articles on IC in neonates and infants, emergencies and specific clinical circumstances including infection by human immunodeficiency virus, surgery, disability, termination of pregnancy, contraception, ethical dilemmas or in languages other than English or Spanish.
Selection stageWe assessed eligible sources through the critical reading of the titles and abstracts, classifying them by topic into 3 categories: Law and ethics of IC in minors; content and readability of IC forms and assessment of competence in the minor. We then performed an additional screening based on the following criteria:
Inclusion criteria:
- -
Review articles and reports related to international law and ethical guidelines on the age of consent or assent in minors and international regulations on paediatric research.
- -
Original articles corresponding to studies that assessed tools for the evaluation of comprehension skills and decision-making capacity in minors including data on sample characteristics, intervention, outcome variables and results that would allow a descriptive analysis and comparison with data from other selected studies.
Exclusion criteria:
- -
Studies and bioethics dissertations on the attitudes of minors toward IC.
- -
Studies based on focus groups of minors that did not include objective assessment tools.
- -
Case studies, brief articles or letters to editors.
- -
Articles on content and readability of IC.
- -
Inclusion stage.
- -
Analysis of selected articles.
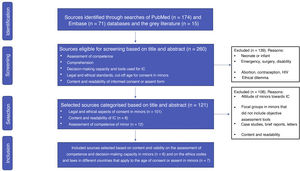
Fig. 1 presents a flow chart of the process of article selection. Of the 260 sources found in PubMed (n = 174), Embase (n = 71) and the grey literature (n = 15), we excluded 139 for meeting 1 or more of the exclusion criteria. After classifying the remaining 121 articles, we finally included 13 applying the criteria established for the selection stage.
Review of legislation on consent in minorsTable 1 presents the selected articles focused on the guidelines and regulations that define the role of the minor in decisions concerning health care and/or research at the international level, analysing the regulatory framework of countries with different levels of development and health resources.
Comparison of regulations on IC for decisions regarding health care and/or research in minors.
| Author/year | Country | Law/guideline | Consent to health care/research by minor |
|---|---|---|---|
| Mullins-Owens et al., 201214 | USA (FDA)China (SFDA)Kenya (NCST)India (ICMR) | Code of Federal Regulation 46 102 (i) 2009Biomedical Research involving human Ethics Review Principles art. XIV (F) 2007Guidelines for Ethical conduct of Biomedical Research involving human subjects in Kenya (2005)Ethical Guidelines for Biomedical research on human participants, ICMR guidelines (2006) | Requires assent of minor and consent of legal representative. Inconsistencies in age and process due to disparities between federal regulation and the guidelines of ethics committeesNot expressly requiredNot expressly required. The dissent of the minor is respected unless there is no alternative treatmentNot expressly required |
| Coleman and Rosoff, 201315 | USAa | Based on state law, statutes or legal precedent | Differences between states. Fourteen allow minors to consent under specific circumstances if mature enough, and 3 based solely on age or maturityDifferent age minimums applied: 14, 15, 16 or 18 yearsIn some states, a specific age is not established, but is based on the maturity of the minor |
| Stultiëns et al, 200716 | EU member states that ratified European Convention on Human Rights and Biomedicineb | Each member state establishes domestic regulations | Four distinct situations:a) Lawful medical decision-making capacity = age of legal majority (18 years): Cyprus, Greece and Slovakiab) Lawful decision-making capacity < age of legal majority: Denmark and Slovenia (15 y), Spain (16 y), Portugal (14 y)c) Lawful decision-making capacity evaluated on a case-by-case basis, according to age and maturity: Czech Republic and Estoniad) Both b + c: Lithuania |
| Palazzani et al, 201717 | SpainItalyUnited Kingdom (UK)GermanyFranceAustria | Transposition of 2001/20/EC directive to national legislation and domestic lawAdaptation of domestic law to Regulation (EU) 536/2014 on clinical trials | Different provisions regarding assent/consent from minor:a) UK, Italy, France: not expressly requiredb) Spain and Austria: required at 12 and 14 years, respectivelyc) Germany: required based on ability of the minor to understand research (case-by-case)Different age-based criteria:a) UK and Spain: 16 and 12 years, respectivelyb) Italy, Germany, Austria, France: 18 years (or 16 in France in the case of legal emancipation)Different definition of mature minor:a) UK and France: 16 years (and emancipated minors in France), competent to consent to participation in clinical trials.b) Italy and Germany: case-by-case basis, based on maturity.c) Austria and Spain: 14 and 12 years, respectively |
| Pinto Bustamante and Gulfo-Díaz18 | Colombia | Law 1098 of 2006 (code on children and adolescents), sentence T-411 of 1994, Sentence T-477 of 1995, Sentence T-474 of 1996, Sentence C-900 of 2011 | Does not establish provisions for medical emancipation nor a specific age for consent. Lawful consent can be provided for research, but not for health care. Definition of mature minor: 14−17 years. Limited lawful capacity to consent to medical interventions and make medical decisions under specific circumstances |
| Parra Sepúlveda and Ravetllat Ballesté19 | Chile | Law no. 20.584 | Age: 14−17 years. Consent that is not legally binding. The minor can express the will to consent to or refuse medical intervention |
| Casanova Torrado20 | Uruguay | Decree 274/2010 (informed consent)Law 17.823 (Code on Children and Adolescents)Law 18.335 (rights and responsibilities of patients)Medical Ethics Code | No specific age. Based on the maturity and competence of the minor. If the physician considers that the minor is competent, IC does not need to be obtained from a legal guardian (guardian only needs to be informed) |
Mullins et al. concluded that the regulatory framework for minors in clinical research has been developed more thoroughly in the United States and in Europe, where the bulk of paediatric trials take place, while in developing countries such as China, India and Kenya guidelines and regulations apply mainly to the general population, leaving a regulatory vacuum in children and adolescents. In these countries, cultural factors play an important role in the perception of the capacity of minors to consent.
In China, regulations on decision-making contemplate the assent of the family—autonomy is perceived rather as a collective versus as individual right—while in Kenya the approach is more paternalistic and access to treatment may be granted through participation in clinical trials, at times unbeknownst to the patient. There is little opportunity for the enforcement of laws guaranteeing the protection of children in research due to socioeconomic factors.
Guidelines in India (ICMR) call for taking precautions and minimising risk for the general population, without establishing specific guidelines for children, in contrast with the United States, which requires that the study protocol offer a potential direct benefit to the minor and that research involves the minimum possible risk to paediatric participants or a slight increase above the minimum possible risk. Most states do not allow minors to give consent to health care and, while there are exceptions to this rule, they require evidence of the maturity and decision-making capacity of the minor, even if formal evaluation of these aspects is not required.
A comparison of legal regulations regarding the role of minors in medical decision-making in the European Union performed by Stultiëns et al. analysed the legislation applicable to minors in 9 of the member states that ratified the European Convention on Human Rights and Biomedicine. The authors found variations in domestic law between states and in the age and circumstances under which minors could make health care decisions autonomously.
A comparison of regulations regarding IC in minors in Europe and in the United States showed that European states grant greater autonomy to minors in decisions concerning their health. The explanatory report of the Convention stated that “in certain situations which take account of the nature and seriousness of the intervention as well as the minor’s age and ability to understand, the minor’s opinion should increasingly carry more weight in the final decision”.
An analysis of European legislation by Palazzani et al. found heterogeneity in the laws that determine the age at which minors can provide lawful consent to participate in research. Every country requires consent from the legal representative of the minor for proxy consent and decision-making, and the age at which the minor can give consent/assent may not be the same as the legal age of majority, as is the case of Spain, Austria and United Kingdom. The rest of the member states included in the analysis did not establish an age limit: the competency to assent was determined based on the maturity of the minor as assessed by the researcher, and thus established on a case-by-case basis.
In Latin America, regulations on consent by minors have evolved toward a greater recognition and promotion of autonomy in medical decision-making. Each of the countries analysed (Uruguay, Colombia and Chile) has developed domestic regulations incorporating different elements and based on a shared code of bioethics that establishes children as subjects of rights. Some countries allow autonomous consent by adolescents not yet of legal age if the health professional considers them sufficiently mature.
Assessment of the competence of minors in informed consent in medical research or decision-makingTable 2 presents the studies devoted to the assessment of the competence of minors for IC in medical research or health care decision-making with an objective instrument. Four of these studies used the semi-structured MacArthur Competency Assessment Tool for Clinical Research adapted for children and adolescents (MacCAT-T, MacCAT-CR).
Comparison of studies that analysed the competence of minors: understanding and decision-making capacity.
| Author, yearStudy design | Study universe | Sample size/age range (years) | Country | Competence assessment tool | Conclusions of assessment of informed consent capacity (for research or health care-related decisions) |
|---|---|---|---|---|---|
| Hein et al, 201521Pilot study | Paediatric outpatients at risk for an autosomal dominantly inherited cardiac disease (eligible for predictive genetic testing) | N = 176-18 | Netherlands | MacCAT-T (MacArthur Competence Assessment Tool for Treatment) | The MacCAT-T is appropriate to assess capacity for consent in this populationChildren aged < 10 years considered not competentChildren aged 12−18 years considered competent to provide IC |
| Nelson et al, 201622Pilot study | Outpatient community-based sample | N = 3014-21 | United States | MacCAT-CR (MacArthur Competence Assessment Tool for Clinical Research)REALM (Rapid Estimate of Adult Literacy in Medicine) to assess health literacy | The MacCAT-CR adapted for use in adolescents is feasible and its results are significantly associated with health literacy and socioeconomic status |
| Koelch et al, 200923Feasibility study | Psychiatric patients with attention deficit/hyperactivity disorder (ADHD) or ADHD combined with oppositional defiant disorder (ODD) that participated in 2 clinical trials | N = 197-15 | Germany | MacCAT-CR (MacArthur Competence Assessment Tool for Clinical Research) | Individuals aged > 12 years (compared to 9−12 years) demonstrated competence in relation to practical aspects of research: procedures, discomfort, drawbacks. Good understanding of the principle of autonomyWhen it came to abstract concepts, such as the primary purpose of the trial or the nature of placebo, they exhibited a poor level of appreciation, comparable to that of parents.This suggests that the information provided for IC regarding the most abstract aspects of clinical trials needs to be more extensive and preciseEvidence suggestive of therapeutic misconception in both minors and parentsThe findings suggest that the MacCAT-CR is a consistent instrument but requires further development and adaptation |
| Hein et al, 201424Prospective study | Children and adolescents managed in the inpatient and outpatient departments of allergology, gastroenterology, oncology, ophthalmology eligible for clinical research studies | N = 1616-18 | Netherlands | MacCAT-CR (MacArthur Competence Assessment Tool for Clinical Research) | The MacCAT-CR scale exhibited robust psychometric properties for assessment of decision-making capacity in minors and a strong correlation with ageChildren aged 9–11 years, consent may be justified when competence can be demonstrated in individual cases by the MacCAT-CR |
| Espejo et al, 201125Cross-sectional, observational quantitative study | Adolescents enrolled in years 3 and 4 of compulsory secondary education in Spain (ESO) in rural and urban settings | N = 6014-15 | Spain | Escala de Valoración de Competencia (Competence Assessment Scale, EVC)Rest’s Defining Issues Test (DIT): moral developmentLikert scales: assessment of cognitive development and maturity (ACDM) | The EVC could be valid and reliable for assessing moral competence in the context of medical decision-making in adolescents aged 14–15 yearsHigh correlation between EVC and ACDM scales. No correlation between EVC and DIT |
| Gibson et al, 201126Qualitative descriptive study | Researchers with 3−30 years of experience in paediatric research | N = 14Not applicable | Canada | Qualitative analysis of face-to-face interviews with researchers | Authors propose involving the family (both minors and parents, preferably simultaneously) in the consent processThe consent capacity of the minor is not assess routinely and the ability to consent is frequently judged based solely on age or existing guidelines |
Although there were limitations concerning sample size and the study design in 3 of the 4 studies that applied this tool, all found the instrument feasible in children, consistent psychometric properties and adequate reliability and precision in establishing competence in the minor.
Use of this tool has evinced sufficient competence for IC in minors starting from age 12 years, with minors with attention-deficit disorder or attention-deficit hyperactivity disorder actually exhibiting a higher level of comprehension compared to parents, according Koelch et al. The study by Nelson et al. in youth aged 14–21 years, age was one of the independent predictors of the MacCAT score along with health literacy and socioeconomic status. The prospective study conducted by Hein et al. in 160 patients aged 6–18 years, inpatients and outpatients, concluded that age was a good predictor of competence based on the MacCAT-CR score. The authors also concluded that autonomous consent was warranted in children aged 9–11 years who could demonstrate their personal competence by means of the MacCAT-CR.
The study by Espejo et al., conducted in Spain, assessed the competence of students for consent to health care interventions using the Lleida Competence Assessment Scale (Escala de Valoración de la Competencia [EVC]), the Defining Issues Test developed by James Rest to assess moral development and 2 Likert scales to assess cognitive skills and maturity. The study concluded that the EVC was an adequate instrument to assess moral competence in students aged 14 and 15 years and could be used in specific clinical situations, taking into account that there are other factors or situations that may affect its applicability, such as emergencies or disease severity.
Last of all, the descriptive study by Gibson et al. assessed the knowledge and skills of researchers regarding consent and capacity in the context of obtaining IC for participation of minors in research protocols. The authors found substantial heterogeneity in the perceptions of researchers and discrepancies between ethical practice and the requirements of ethics boards, in addition to the absence of routine assessment of the capacity of minors, whose ability to provide consent would be determined solely based on the age cut-offs provided in current guidelines. The authors proposed a family-decision model that would allow parents and children to provide consent together and preferably at the same time.
DiscussionThe findings of our review corroborate that despite there being a general international framework of ethics, each country has developed domestic laws regarding when minors can autonomously provide consent and for which health-related decisions.
In the United States and Europe, the concept of the autonomy of the minor is more developed, although most medical interventions require the consent of parents or adult legal representatives of the minors (especially in the USA, due to the widespread practice of defensive medicine).27 Exceptions to this requirement basically apply to voluntary termination of pregnancy and birth control, which in some instances do not require parental consent, and research, where protection of minors is particularly strong. Adults must make decisions based on the best interests of the minor, a fundamental legal principle that entails the contemplation of the specific case and the rights and needs of the minor at hand. At the practical level, this is where conflicts may arise between different stakeholders: clinicians, parents and the minors themselves with severe medical conditions or with no other treatment options, situations whose resolution may require the involvement of a health care ethics committee.28
The situation is different in developing countries where the practice of clinical research is relatively new, family and parents may play a more important role due to cultural, social and health care-related factors and clinical trials may be the only way to access certain treatments. However, Latin American countries, whose legislation was included in our review, have laws that recognise the rights, autonomy and active participation in decision-making of minors, defining the concepts of best interests of the minor, the mature minor and progressive autonomy29; although Chile and Uruguay would need the development of a specific legal framework for clinical research in minors.
In Europe, the analysis of the 9 states that have ratified the European convention for the protection of human rights and biomedicine reveals significant differences: only 3 (Spain, Denmark and Portugal) establish an age below the age of majority to consent to health care interventions, and another 3 (Czech Republic, Estonia and Lithuania) take into account age and maturity of the patient to determine the capacity for consent in health care.
In Spain, minors can generally consent to health care interventions starting from age 16 years or after legal emancipation, with the exception of clinical trials and assisted reproductive technology procedures, consent for which can only be given after reaching the age of majority,30 and interventions carrying serious risks to health, in which case the onus of decision-making is on the parents, although it is also necessary to consider the opinion of the minor from age 16 years.
After the reform of 2015, the duty to seek and consider the opinion of the minor aged more than 12 years was removed from the law on patient autonomy, although some authors consider that these minors may be sufficiently competent to make decisions about medical treatment in certain cases.24
In the case of clinical trials, IC by the parents or legal representatives of the minor is required (until the age of majority of 18 years) in addition to assent from minors over 12 years (although the legal text does not use this word, but “consent”). Royal decree 1090/2015 also establishes that the opinion of minors under 12 years must be taken into account if they are deemed mature enough.
Age is not the sole factor that determines the decision-making capacity of minors. The assessment of this competence is complex, as maturity is a developmental process that involves the maturation of moral judgment, cognitive development, emotion and the health condition of the minor.25 However, it is reasonable to say that conventional morality develops sometime between 10 and 12 years of age. The findings of our review corroborate that from age 12 years, minors have the capacity to make health-related decisions, as evinced by studies that used the paediatric adaptation of the MacArthur scale. This instrument, which is based on a cognitive model of mental capacity, is a semi-structured interview with items grouped in 4 dimensions: understanding, appreciation, reasoning and expressing a choice. Although this scale is more widely used in adult psychiatric patients,8 it has also proven to be a valid and reliable instrument with consistent psychometric properties in children and adolescents. In addition, there is evidence that this tool offers a high sensitivity and specificity between ages 9.6 and 11.2 years, which suggests that in this age range, as long as the personal capacity to consent of the minor can be demonstrated, consent or assent should be obtained from the minor. Although the EVC scale developed by Espejo et al. could be valid and reliable for the assessment of competence in adolescents, it is restricted by design to decisions regarding health care and has not been used in clinical research or in children under 12 years, so its applicability is more limited.
Few studies to date have used existing instruments to assess competence, and their sample sizes were small, so additional research needs to be conducted at a larger scale on the assessment of competence for consent in minors with the ultimate purpose of providing a solid instrument to researchers and clinicians and to guide changes in legislation on IC.
ConclusionOur review of studies that used instruments validated in the paediatric population in the specific context of medical research showed that the semi-structured MacArthur interview adapted to adolescents is most accurate, thorough and consistent for the evaluation of the minor’s decision-making capacity. While awaiting data from further studies to improve the evidence on the subject, minors aged more than 12 years can be considered competent to make health-related decisions, while the individual decision-making capacity of minors aged 9–12 years can be assessed using the aforementioned instrument.
The legal regulation of IC in paediatric research should take into account the current scientific evidence and establish the assent of the minor as an absolute requirement for recruitment, in every case in individuals aged more than 12 years, and starting from age 9 years if the minor exhibits the capacity to assent.
To assess this capacity in the minor, researchers and clinicians alike should use validated and appropriate tools, such as the semi-structured MacArthur interview, which has proven useful for the purpose.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Clara Ramírez, a GSK scholarship holder in the medical department, for her support in the development of this project.
Please cite this article as: Boceta R, Martínez-Casares O, Albert M. El consentimiento informado en el menor maduro: comprensión y capacidad de decisión. An Pediatr (Barc). 2021;95:413–422.
Previous presentation: The study was presented at the Congress of the Asociación Española de Bioética; October 25–26, 2019; Valencia, Spain.