The aim of this study is to assess epidemiological, clinical and laboratory characteristics of primary infection by Epstein–Barr virus (EBV) in children without previous diagnosis of any immune disease and its relationship with clinical presentation.
Patients and methodsA retrospective study was conducted on all children from 0 to 15 years with IgM against viral capsid of EBV positive or indeterminate during a 22 month period. Epidemiological, clinical and laboratory data were analysed and compared between typical (mononucleosis syndrome) and non-typical clinical symptoms.
ResultsThe study included a total of 103 children, with a median age of 7 years (3–12.5 years). Almost two-thirds (63%) of patients had typical clinical signs, with a mononucleosis syndrome, and 37% had a non-typical presentation. The non-typical clinical group had a lower age (P=.03) and took less antibiotic than the typical clinical group (P=.015). From laboratory studies, there were no differences between the groups, except in RCP, which was higher in typical clinical group (P=.04). Heterophile antibodies were positive in 33% of patients. An indeterminate IgM against viral capsid was present in 20% of the patients, and most of them had an oligosymptomatic or atypical presentation. An IgM positive for other viruses was found in 21%, and 3 of them were suspicious of false positive for EBV.
ConclusionsIn the studied population, a primary infection due to EBV is common in younger ages, and they have usually an oligosymptomatic clinical presentation. A very low percentage of positive heterophile antibodies were found. Cases with indeterminate IgM against viral capsid are more frequent in the non-typical clinical group. Co-infection with other viruses is common.
El objetivo de este estudio es conocer las características epidemiológicas, clínicas y analíticas de la primoinfección por el virus de Epstein-Barr (VEB) en niños sin diagnóstico previo de ninguna enfermedad inmune y su relación con la forma de presentación clínica.
Pacientes y métodosEstudio retrospectivo de pacientes entre 0 y 15 años con IgM sérica frente a la cápside viral del VEB positiva o indeterminada, durante un período de 22 meses. Se analizaron datos epidemiológicos, clínicos y de laboratorio y se compararon según tuvieran una clínica típica (síndrome mononucleósico) o no típica.
ResultadosSe incluyeron 103 niños. La mediana de la edad fue de 7 años (3-12.5 años). El 63% de los pacientes presentaron clínica típica o síndrome mononucleósico y el 37% una clínica no típica. La edad fue significativamente menor en el grupo de clínica no típica (p=0,03) y recibieron menos tratamiento antibiótico (p=0,015). En los parámetros analíticos no hubo diferencias estadísticamente significativas excepto en la PCR, discretamente más elevada en el grupo de clínica típica (p=0,04). El 33% de los pacientes tuvieron anticuerpos heterófilos positivos. El 20% tuvieron una IgM frente a la cápside viral indeterminada, la mayoría con clínica oligosintomática o atípica. El 21% tuvieron IgM positivas para otros virus y 3 de ellos fueron posibles falsos positivos para el VEB.
ConclusionesEn nuestra población, la primoinfección por VEB es frecuente en niños de menor edad, y en ellos predominan las formas oligosintomáticas. El porcentaje de anticuerpos heterófilos positivos ha sido muy bajo en nuestra muestra. Los casos con IgM frente a la cápside viral indeterminada son más frecuentes en el grupo de clínica no típica. Es común detectar coinfección con otros virus.
Primary infection by Epstein–Barr virus (EBV) typically presents with all the main features of mononucleosis: fever, asthenia, enlarged lymph nodes, exudative pharyngitis, hepatosplenomegaly and exanthema following administration of penicillin. But in some cases it presents with few or atypical symptoms, making diagnosis more challenging. In younger patients, primary infection is usually asymptomatic or oligosymptomatic, and it is in adolescence that primary infection starts to present with the full picture of mononucleosis.
The typical age of primary infection by EBV varies based on cultural and socioeconomic factors. In developing countries and rural areas, most children have been infected by EBV between ages 3 and 6 years. In contrast, in developed countries and urban areas primary infection tends to occur at later ages and is more likely to produce more symptoms or a mononucleosis syndrome.1–3
The diagnosis of infectious mononucleosis is based on the clinical presentation supported by abnormal findings of laboratory tests (elevated white blood cell count with lymphocytosis and monocytosis, atypical lymphocytes and elevation of liver enzymes). The presence of heterophile antibodies is highly specific but has a low sensitivity, and their production increases with age.4–6 Oligosymptomatic forms of primary infection by EBV are usually confirmed by serologic testing.
Specific EBV viral capsid antigen (VCA) IgG and IgM antibody tests, EBV early antigen tests and EBV nuclear antigen tests are the serologic tests of choice to diagnose acute infection in immunocompetent hosts and to monitor the course of the infection.5–7 New molecular diagnostic techniques, such as detection of viral DNA by polymerase chain reaction, could help interpret uncertain results of antibody tests, although the published evidence on this approach is still scarce, especially when it comes to healthy individuals and the paediatric population, and further studies are needed to standardise diagnostic viral load values.8–10 These tests are not used to diagnose mononucleosis in immunocompetent patients.11
Few studies have investigated the seroprevalence of EBV and the ages at which primary infection occurs in the Spanish population. One of the studies conducted in Spain, by Pariente et al., found a peak between ages 2 and 4 years and another between ages 14 and 18 years.12
Most of the studies in the literature focus on hospitalised patients with symptoms of infectious mononucleosis,13–15 so few data are available on other forms of primary infection in children without a previous diagnosis of immune disease.
The aim of our study was to establish the epidemiological, clinical and laboratory characteristics of primary infection by EBV in healthy children in our catchment population and to assess their association with the clinical presentation of primary infection.
Patients and methodsStudy design and settingWe performed a retrospective study of the data corresponding to all children aged 0–15 years with positive results of serologic testing for EBV ordered from the primary care system, emergency department or inpatient services.
Sample and selection criteriaWe included patients with positive or indeterminate results of the EBV-VCA IgM test over a period of 22 months (September 2012–June 2014). We included cases identified through the Microbiology Laboratory, which is the regional referral laboratory coordinating services for the public health catchment area of Xátiva-Ontinyent in the province of Valencia, with a population of 204623 inhabitants (2013 census), of who 30636 are aged less than 15 years.
The case definition was positive or indeterminate results of EBV-VCA IgM test using the Liaison® chemiluminescence immunoassay (DiaSorin, Italy). We included cases with indeterminate results because the testing method was quantitative and the detected levels were in a range that was not clearly negative, which could be compatible with the early stages of primary infection. We excluded patients with negative EBV-VCA IgM test results, aged 16 or more years, with an underlying chronic disease or receiving immunosuppressive or steroid therapy.
Data collectionWe collected epidemiological, clinical and laboratory data from electronic health records following the protocol for data access and confidentiality of our hospital. The study was approved by the Clinical Research Ethics Committee of our catchment area.
Variables under studyWe collected data on the following epidemiological variables: sex, age and month of year at time of primary infection. We also collected data on the need for hospital admission and antibiotherapy. As for clinical manifestations, we collected data on the presence of fever, exudative tonsillitis, exanthema, splenomegaly on palpation of the abdomen, lymph node enlargement (>1cm), asthenia and the variable “other symptoms”, which included vomiting, diarrhoea, abdominal pain, jaundice, hepatomegaly on palpation, myalgia and palpebral oedema. Based on these data, we divided the patients into 2 groups. The first group included patients with a typical presentation (mononucleosis syndrome), defined as presence of at least 2 of the following symptoms: fever, pharyngitis, enlarged lymph nodes and asthenia. The non-typical presentation group included the rest of the patients, that is, patients with fewer than 2 of the symptoms listed above (oligosymptomatic patients) and patients with atypical symptoms or symptoms other than those characteristic of the mononucleosis syndrome.
As for laboratory characteristics, we collected data for white blood cell and platelet counts. We also collected the serum levels of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT), defining hypertransaminasaemia as elevation of either by at least twice the normal reference value applied in our laboratory (30IU/mL), serum levels of C-reactive protein (CRP) and, in case of detection of heterophile antibodies, the results of the rapid latex agglutination test on slide (Monogen®, Biokit; Barcelona, Spain). Last of all, we collected data on coinfection with other viruses when additional serologic tests had been ordered.
Statistical analysisWe used the statistical software SPSS® version 21.0 to analyse the data. We performed a descriptive summary and compared the groups with typical and non-typical presentations. We have summarised quantitative variables as median and interquartile range (IQR), and qualitative variables as absolute and relative frequencies.
We used the Fisher exact test to compare qualitative variables and the Kruskal–Wallis test to compare quantitative variables (which did not follow a normal distribution). We defined statistical significance as a P-value of .05 or less in any of the tests.
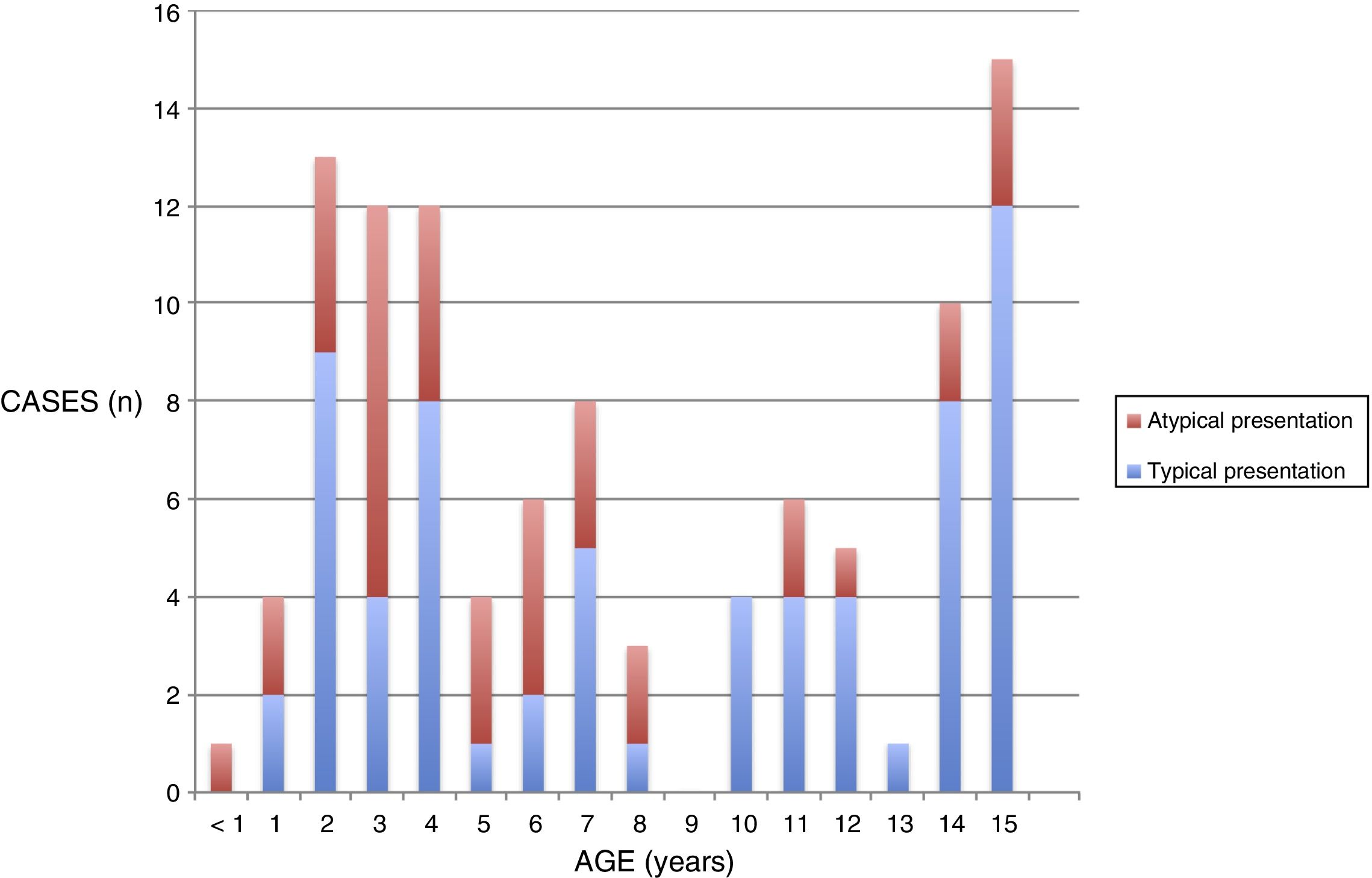
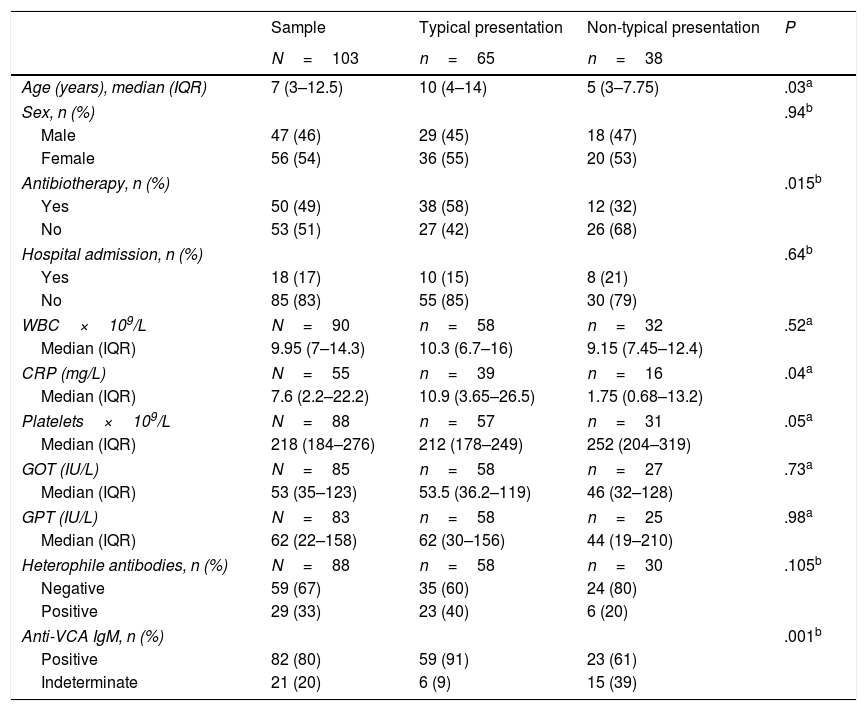
ResultsDescriptive analysisThe sample included 103 children. The sex distribution was 47% male and 56%, female. The median age of the patients was 7 years (IQR, 3–12.5). We found 3 age groups in which the incidence peaked: 2–4 years, 7 years and adolescence (14–15 years) (Fig. 1).
Sixty-two percent of the sample acquired the primary infection in autumn or winter (September–February).
In every case, serological tests were ordered due to clinical suspicion of primary infection. The most frequent suspected diagnoses that led to ordering of serologic tests were infectious mononucleosis (22%), lymphadenitis (18%) and tonsillitis (11%). Most serologic tests were ordered in the hospital (44% in the emergency department and 34% in the inpatient paediatric ward or outpatient paediatric clinic). The remaining 22% were ordered at the primary care level.
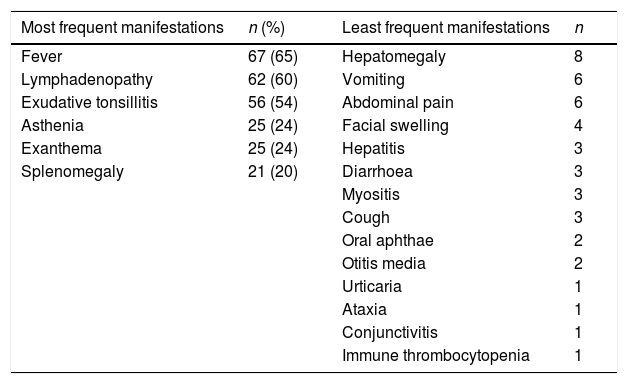
Table 1 summarises the clinical manifestations documented in the electronic health records.
Clinical characteristics of the sample (N=103).
| Most frequent manifestations | n (%) | Least frequent manifestations | n |
|---|---|---|---|
| Fever | 67 (65) | Hepatomegaly | 8 |
| Lymphadenopathy | 62 (60) | Vomiting | 6 |
| Exudative tonsillitis | 56 (54) | Abdominal pain | 6 |
| Asthenia | 25 (24) | Facial swelling | 4 |
| Exanthema | 25 (24) | Hepatitis | 3 |
| Splenomegaly | 21 (20) | Diarrhoea | 3 |
| Myositis | 3 | ||
| Cough | 3 | ||
| Oral aphthae | 2 | ||
| Otitis media | 2 | ||
| Urticaria | 1 | ||
| Ataxia | 1 | ||
| Conjunctivitis | 1 | ||
| Immune thrombocytopenia | 1 |
We classified patients based on their clinical presentation. Of all patients, 63% (n=65) had a typical presentation of mononucleosis syndrome, and 37% (n=38) a non-typcial presentation.
Fifty patients (49% of the total) received antibiotic treatment (penicillins and penicillin derivatives) at some point during the course of disease; 19 out of the 50 (38%) developed exanthema, whereas only 6 of the 53 not treated with antibiotics (11%) developed this symptom (P=.002).
Seventeen percent of the patients (n=18) required admission to the hospital, without differences based on the type of presentation. The most frequent reasons for admission were general malaise, prolonged duration of symptoms, laboratory abnormalities (thrombocytopenia, leukopenia) and the presence of non-typcial features such as myositis or ataxia.
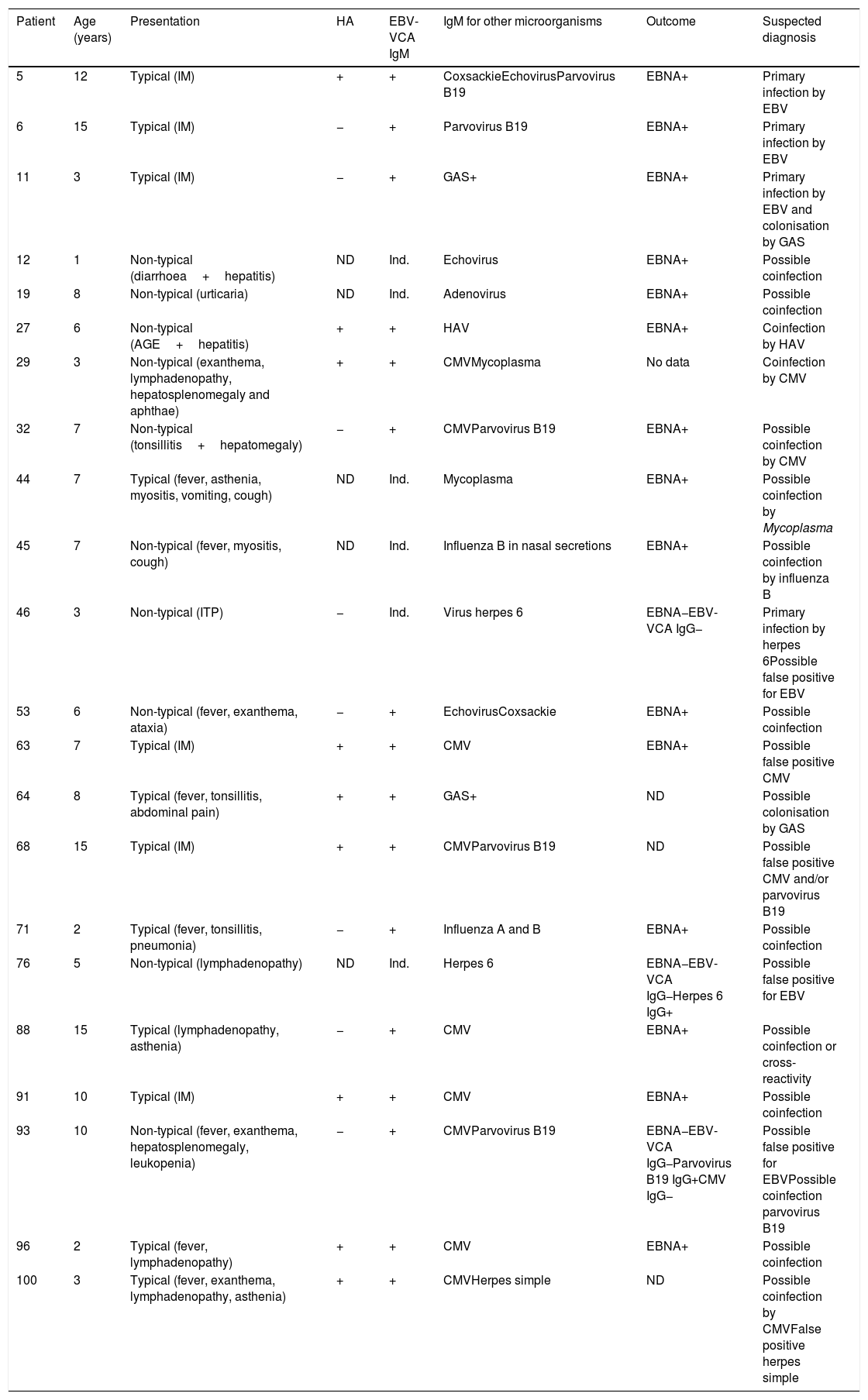
In 22 patients (21%) that underwent serologic testing for other organisms, the results of specific IgM tests were positive for another virus, with detection of more than one virus or other microorganisms in some of them. The most frequent coinfections involved cytomegalovirus (CMV) and parvovirus B19. Tests for detection of DNA of other viruses were not ordered in any case. Table 2 summarises the clinical features and serologic characteristics of these patients, as well as the outcomes of those for who we were able to obtain the data.
Patients with positive serologic tests for other infectious agents.
| Patient | Age (years) | Presentation | HA | EBV-VCA IgM | IgM for other microorganisms | Outcome | Suspected diagnosis |
|---|---|---|---|---|---|---|---|
| 5 | 12 | Typical (IM) | + | + | CoxsackieEchovirusParvovirus B19 | EBNA+ | Primary infection by EBV |
| 6 | 15 | Typical (IM) | − | + | Parvovirus B19 | EBNA+ | Primary infection by EBV |
| 11 | 3 | Typical (IM) | − | + | GAS+ | EBNA+ | Primary infection by EBV and colonisation by GAS |
| 12 | 1 | Non-typical (diarrhoea+hepatitis) | ND | Ind. | Echovirus | EBNA+ | Possible coinfection |
| 19 | 8 | Non-typical (urticaria) | ND | Ind. | Adenovirus | EBNA+ | Possible coinfection |
| 27 | 6 | Non-typical (AGE+hepatitis) | + | + | HAV | EBNA+ | Coinfection by HAV |
| 29 | 3 | Non-typical (exanthema, lymphadenopathy, hepatosplenomegaly and aphthae) | + | + | CMVMycoplasma | No data | Coinfection by CMV |
| 32 | 7 | Non-typical (tonsillitis+hepatomegaly) | − | + | CMVParvovirus B19 | EBNA+ | Possible coinfection by CMV |
| 44 | 7 | Typical (fever, asthenia, myositis, vomiting, cough) | ND | Ind. | Mycoplasma | EBNA+ | Possible coinfection by Mycoplasma |
| 45 | 7 | Non-typical (fever, myositis, cough) | ND | Ind. | Influenza B in nasal secretions | EBNA+ | Possible coinfection by influenza B |
| 46 | 3 | Non-typical (ITP) | − | Ind. | Virus herpes 6 | EBNA−EBV-VCA IgG− | Primary infection by herpes 6Possible false positive for EBV |
| 53 | 6 | Non-typical (fever, exanthema, ataxia) | − | + | EchovirusCoxsackie | EBNA+ | Possible coinfection |
| 63 | 7 | Typical (IM) | + | + | CMV | EBNA+ | Possible false positive CMV |
| 64 | 8 | Typical (fever, tonsillitis, abdominal pain) | + | + | GAS+ | ND | Possible colonisation by GAS |
| 68 | 15 | Typical (IM) | + | + | CMVParvovirus B19 | ND | Possible false positive CMV and/or parvovirus B19 |
| 71 | 2 | Typical (fever, tonsillitis, pneumonia) | − | + | Influenza A and B | EBNA+ | Possible coinfection |
| 76 | 5 | Non-typical (lymphadenopathy) | ND | Ind. | Herpes 6 | EBNA−EBV-VCA IgG−Herpes 6 IgG+ | Possible false positive for EBV |
| 88 | 15 | Typical (lymphadenopathy, asthenia) | − | + | CMV | EBNA+ | Possible coinfection or cross-reactivity |
| 91 | 10 | Typical (IM) | + | + | CMV | EBNA+ | Possible coinfection |
| 93 | 10 | Non-typical (fever, exanthema, hepatosplenomegaly, leukopenia) | − | + | CMVParvovirus B19 | EBNA−EBV-VCA IgG−Parvovirus B19 IgG+CMV IgG− | Possible false positive for EBVPossible coinfection parvovirus B19 |
| 96 | 2 | Typical (fever, lymphadenopathy) | + | + | CMV | EBNA+ | Possible coinfection |
| 100 | 3 | Typical (fever, exanthema, lymphadenopathy, asthenia) | + | + | CMVHerpes simple | ND | Possible coinfection by CMVFalse positive herpes simple |
AGE, acute gastroenteritis; CMV, cytomegalovirus; EBNA IgG, IgG against Epstein–Barr nuclear antibody; EBV, Epstein–Barr virus; EBV-VCA IgG, IgG against Epstein–Barr virus viral capsid antigen; EBV-VCA IgM, IgM against Epstein–Barr virus viral capsid antigen; GAS, group A Streptococcus pyogenes; HA, heterophile antibodies; HAV, hepatitis A virus; IM, infectious mononucleosis; Ind., indeterminate; ITP, idiopathic thrombocytopenic purpura; ND, not documented.
As for the laboratory parameters (Table 4), one patient had leukopenia (<2×109/L) and another severe thrombocytopenia (2×109/L). The latter received a diagnosis of idiopathic thrombocytopenia purpura and had coinfection by herpes simplex virus 6. The patient was treated with intravenous immunoglobulin. The peak CRP level was 121.5mg/L, with no evidence in culture of bacterial superinfection.
Hypertransaminasaemia was detected in 51% of the patients (n=42), with a maximum GOT value of 2.236IU/mL and a maximum GPT value of 3.347IU/mL. Only 3 of these patients exhibited symptoms of acute hepatitis (jaundice, vomiting, abdominal pain), and coinfection was found in 2 of them (echovirus in patient 12 and hepatitis A in patient 27).
In 88 patients, a heterophile antibody test was ordered at the time of diagnosis, with positive results in 29 cases (33%).
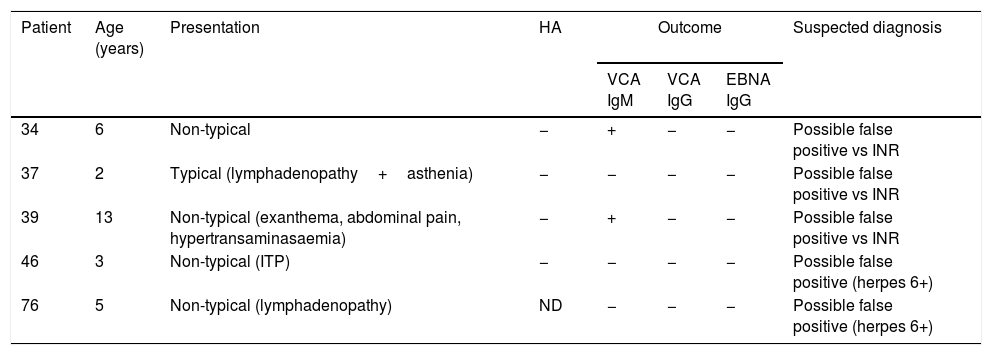
When it came to serologic testing, 20% of the patients (n=21) had indeterminate results of IgM tests at the time of primary infection. Of these patients, 7 were in the typical presentation group, although only 2 exhibited the full picture of infectious mononucleosis, while the rest were oligosymptomatic (2 symptoms). The remaining 14 were classified in the non-typical presentation group, with 7 exhibiting atypical manifestations (hepatitis, urticaria, aphthae, myositis and thrombocytopenia) and the remaining 7 being oligosymptomatic (1 symptom). In 5 cases, heterophile antibodies were not detected and the EBV-VCA IgG and EBV nuclear antigen IgG tests were negative during the follow-up (Table 3).
Patients with indeterminate results of viral capsid antigen IgM test at the time of primary infection and no subsequent seroconversion.
| Patient | Age (years) | Presentation | HA | Outcome | Suspected diagnosis | ||
|---|---|---|---|---|---|---|---|
| VCA IgM | VCA IgG | EBNA IgG | |||||
| 34 | 6 | Non-typical | − | + | − | − | Possible false positive vs INR |
| 37 | 2 | Typical (lymphadenopathy+asthenia) | − | − | − | − | Possible false positive vs INR |
| 39 | 13 | Non-typical (exanthema, abdominal pain, hypertransaminasaemia) | − | + | − | − | Possible false positive vs INR |
| 46 | 3 | Non-typical (ITP) | − | − | − | − | Possible false positive (herpes 6+) |
| 76 | 5 | Non-typical (lymphadenopathy) | ND | − | − | − | Possible false positive (herpes 6+) |
EBNA IgG, IgG against Epstein–Barr nuclear antigen; HA, heterophile antibodies; INR, immunological nonresponder; ITP, idiopathic thrombocytopenic purpura; ND, not documented; VCA IgG, IgG against viral capsid antigen; VCA IgM, IgM against viral capsid antigen.
We compared the different variables in the two clinical presentation groups. Patients in the non-typicalpresentation group were younger (P=.03) (Fig. 1).
In the typical presentation group, treatment with antibiotics was more frequent (P=.015). The levels of CRP were also slightly more elevated in this group (P=.04). There were no other significant differences between the groups in the results of blood tests. Positive EBV-VCA IgM results at diagnosis were more frequent in the typical presentation group, whereas indeterminate results of the test were more frequent in the non-typical presentation group (P=.001) (Table 4).
Clinical and laboratory characteristics by type of presentation of primary infection.
| Sample | Typical presentation | Non-typical presentation | P | |
|---|---|---|---|---|
| N=103 | n=65 | n=38 | ||
| Age (years), median (IQR) | 7 (3–12.5) | 10 (4–14) | 5 (3–7.75) | .03a |
| Sex, n (%) | .94b | |||
| Male | 47 (46) | 29 (45) | 18 (47) | |
| Female | 56 (54) | 36 (55) | 20 (53) | |
| Antibiotherapy, n (%) | .015b | |||
| Yes | 50 (49) | 38 (58) | 12 (32) | |
| No | 53 (51) | 27 (42) | 26 (68) | |
| Hospital admission, n (%) | .64b | |||
| Yes | 18 (17) | 10 (15) | 8 (21) | |
| No | 85 (83) | 55 (85) | 30 (79) | |
| WBC×109/L | N=90 | n=58 | n=32 | .52a |
| Median (IQR) | 9.95 (7–14.3) | 10.3 (6.7–16) | 9.15 (7.45–12.4) | |
| CRP (mg/L) | N=55 | n=39 | n=16 | .04a |
| Median (IQR) | 7.6 (2.2–22.2) | 10.9 (3.65–26.5) | 1.75 (0.68–13.2) | |
| Platelets×109/L | N=88 | n=57 | n=31 | .05a |
| Median (IQR) | 218 (184–276) | 212 (178–249) | 252 (204–319) | |
| GOT (IU/L) | N=85 | n=58 | n=27 | .73a |
| Median (IQR) | 53 (35–123) | 53.5 (36.2–119) | 46 (32–128) | |
| GPT (IU/L) | N=83 | n=58 | n=25 | .98a |
| Median (IQR) | 62 (22–158) | 62 (30–156) | 44 (19–210) | |
| Heterophile antibodies, n (%) | N=88 | n=58 | n=30 | .105b |
| Negative | 59 (67) | 35 (60) | 24 (80) | |
| Positive | 29 (33) | 23 (40) | 6 (20) | |
| Anti-VCA IgM, n (%) | .001b | |||
| Positive | 82 (80) | 59 (91) | 23 (61) | |
| Indeterminate | 21 (20) | 6 (9) | 15 (39) | |
Anti-VCA IgM, IgM against viral capsid antigen; CRP, C-reactive protein; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; WBC, white blood cells.
In our study, we found 2 age peaks in the incidence of primary infection by EBV before adolescence: 2–4 years and 7 years. Historically, the literature has described a higher incidence in adolescence.2 In our sample, up to 64% of patients with a serologic diagnosis of primary infection by EBV were aged less than 10 years, probably because older children have a more florid presentation, which in many cases leads to a clinical diagnosis without the ordering of confirmatory laboratory tests that was the basis of our study.14–16
Despite the higher frequency in younger children, our study found a predominance of the typical presentation (65 cases). In the group of patients with typical features, 13 only had 2 symptoms and 52 more than 2 symptoms. However, in many instances the third symptom belonged to the composite variables “other symptoms”, which encompassed clinical manifestations that are not specifically part of mononucleosis syndromes. This approach to the classification of clinical features may have led to an overestimation of the frequency of typical presentations in our sample.
The most frequent clinical manifestations at the time of primary infection by EBV were fever, cervical lymphadenopathy and exudative tonsillitis, which was consistent with most other published case series,3,13–15,17 although with different proportions. These differences are probably due to our sample including children that were not hospitalised, unlike most other studies in the reviewed literature.
One salient finding of our study was that coinfection by other viruses was detected in 22% of the sample. In some of these cases it is difficult to identify the aetiological agent based solely on the clinical presentation. After studying the outcomes in these children, we considered that in the cases that a follow-up evaluation was requested and patients had positive results of the EBV antinuclear antigen IgG test, the patients may have had coinfection in addition to primary infection with EBV, although we were unable to confirm this with certainty with the available data. There is a greater certainty that cases with typical presentations and positive for heterophile antibodies are cases of coinfection. There are few references in the literature about coinfection with other viruses, although there have been published reports of cases with serologic tests positive for both EBV and CMV.13,18 There are also 2 published cases with severe thrombocytopenia and coinfection by herpes simplex 6 and CMV,18,19 similar to patient 46 in our sample.
We considered the cases of patients 46, 76 and 93 possible false positives for EBV, although we were unable to confirm this, as DNA testing for detection of EBV was not performed at the time of diagnosis of primary infection and some individuals may not mount an immune response following primary infection.
The cases with indeterminate results of EBV-VCA IgM tests are also difficult to interpret, especially in the context of an atypical presentation. Of the patients for who we could obtain follow-up data, 5 did not seroconvert (Table 3). Patients 46 and 76 had positive IgM tests for other viruses (herpes simplex 6), and taking into account their presentation and the negative results of the heterophile antibody tests, we believe that these cases were probably false positives for EBV. In contrast, in patients 34 and 39, the EBV-VCA IgM test became positive after the initial test, and no other data were recorded suggesting coinfection, so we cannot tell whether they represented false positives for EBV or just had not developed immunological memory against the virus. None had complications and there was no evidence supporting the possibility of an underlying immune disorder at the time, although patient 39 had a history of lymphoblastic leukaemia diagnosed 6 years before that was currently in remission without treatment. It is important not to rule out the diagnosis of primary infection in patients with indeterminate results of IgM tests, as this is a possible occurrence in the early stage of infection. It may also be helpful to rule out coinfection by herpes viruses and repeat the serologic test later on in cases with indeterminate EBV-VCA IgM test results to see if there had been changes in serologic status.
The percentage of patients that tested positive for heterophile antibodies (33%) was lower in both groups compared to the current literature. Authors such as Sumaya and Ench found a prevalence of heterophile antibodies of up to 83% in children aged more than 4 years; although this proportion dropped to 50% in children aged 2 and 3 years.4,6 Other European authors have reported a somewhat lower prevalence (42%), in addition to a frequent proportion of negative tests in the early and late stages of primary infection.20 A subsequent review, however, has reported positive rates of 25–50% in children aged less than 12 years,21 which is more consistent with the prevalence found in our study. It is also worth noting that the different techniques used to detect heterophile antibodies do not have the same sensitivity and specificity.22 The test used in our laboratory (Monolatex, Biokit) has a sensitivity of 76% and a specificity of 98% according to the manufacturer. The low production of heterophile antibodies in our sample maybe due to 64% of the patients being less than 10 years of age. Another possible explanation is that tests may have been ordered in the early or the late stage of primary infection, especially in the 22% that were ordered in primary care clinics. Other Spanish authors have also found a low prevalence (32%) of heterophile antibodies in children aged more than 4 years without a clear explanation.13
Patients with oligosymptomatic forms of disease tended to be younger, as has been described in the literature.1–3 We did not find significant differences in the rest of the epidemiological and laboratory characteristics between both groups, although the number of patients for which we had data for some variables was small. We did not find descriptive studies in the literature on the different clinical presentations of primary infection. Most published studies have focused on hospitalised patients and patients with a presentation of infectious mononucleosis.13,14,17
Our study has the limitations inherent in retrospective designs, as we could not obtain follow-up data that could have helped interpret test results in some cases. We are aware of the potential bias involved in not being able to include the entire population of children with EBV infection, as we could only identify those that presented at least 1 symptom and in whom serologic tests were ordered for confirmation. Furthermore, the non-typical presentation group included patients that could have exhibited divergent patterns. On one hand, there were oligosymptomatic patients in whom one would expect a benign course of disease. On the other, there are patients with atypical manifestations, in whom we cannot rule out that the atypical presentation was due to some impairment in the immune response. At any rate, none of the patients with non-typical manifestations developed complications, and all had favourable outcomes.
On the other hand, we have managed to exclude the source of bias involved in having a sample consisting solely of hospitalised patients, and included patients with oligosymptomatic and atypical presentations.
ConclusionsIn our catchment population, primary infection by EBV mainly occurs in younger children, with a predominance of oligosymptomatic presentations. The percentage of patients that tested positive for heterophile antibodies was very low in both the typical and the non-typical presentation groups, with positive results being more frequent in patients with a typical presentation. Cases with indeterminate results of the EBV-VCA IgM test were more frequent in the non-typical presentation group, in both oligosymptomatic patients and patients with atypical features.
Coinfection by other viruses is common, most frequently by CMV and parvovirus B19; in these cases, it is advisable to rule out a false positive.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: García-Peris M, Jiménez Candel MI, Mañes Jiménez Y, Pariente Martí M, González Granda D, Calvo Rigual F. Primoinfección por el virus de Epstein-Barr en niños sanos. An Pediatr (Barc). 2019;90:376–385.
Previous presentation: This study was presented as a poster (“Evolución serológica de la primoinfección por VEB en niños sanos”) at the 62 Extraordinary Congress of the Asociación Española de Pediatría; June 2014, Madrid, Spain. It was also presented as an oral communication (“Primoinfección sintomática por el VEB: más frecuente de lo que parece”) at the XXXI Meeting of the Sociedad Valenciana de Pediatría; June 2015, Castellon, Spain.