Neonatal jaundice is common, especially in premature infants. Compliance treatment protocols with standard serum bilirubin curves force the clinician to separate the child from the mother after birth for phototherapy. The objective of this study is to evaluate the effectiveness and safety of two innovative devices for phototherapy including a LED light mesh: one sleeping bag and one blanket compared to conventional hospital or ambulatory phototherapy.
MethodsTwo randomised clinical trials were conducted: one with newborns >2000 g at birth in the Neonatal Care Unit and the other with premature infants followed-up in an outpatient Kangaroo Mother Care (KMC) programme. The gold standard for bilirubin measurement was serum bilirubin, and ambulatory controls were performed with the Bilicheck®. Parents and health personnel completed a questionnaire on comfort and perceptions.
ResultsIn the study using the bag, a linear regression was performed for the decrease in bilirubin in mg/dL/h, controlling by early jaundice (<36 h) and the device type. The results were similar between the two devices. For the blanket trial in the KMC programme, the decrease in bilirubin levels with the new device was significantly greater. No differences in temperatures, duration of phototherapy, re-admission, mortality, or side effects for both trials were found. Parents and staff satisfaction with the two devices was identical for the two trials.
ConclusionThese two small studies add a "grain of sand" to humanisation of newborn care, avoiding the mother-and-child separation for both the intra-hospital high-risk hyperbilirubinaemia, as well as for the lower-risk hyperbilirubinaemia in an outpatient clinic.
La ictericia neonatal es frecuente en los prematuros. El tratamiento oportuno actual con curvas estándar para fototerapia lleva al clínico a separar el bebe de su madre después del nacimiento. Se busca evaluar la efectividad y seguridad de dos dispositivos innovadores para fototerapia compuestos por mallas de luz LED: una bolsa para dormir y una manta comparadas con fototerapia convencional intrahospitalaria o ambulatoria.
MétodosSe condujeron dos experimentos clínicos aleatorizados: uno con neonatos > 2000 g en una unidad de recién nacidos y otro con prematuros en un programa de seguimiento ambulatorio. El patrón de oro para la medición de bilirrubinas fue la bilirrubina sérica y los controles ambulatorios fueron hechos con el Bilicheck®. Padres y personal de salud respondieron una encuesta sobre comodidad con los dispositivos.
ResultadosEn el estudio de la bolsa se realizó regresión lineal para descenso de bilirrubinas en mg/dl/h, controlando por ictericia de aparición temprana (<36 h) y tipo de dispositivo. No se encontró diferencia significativa entre los dispositivos. Para el estudio en el seguimiento ambulatorio el descenso de bilirrubina con la manta fue significativamente mayor. En ambos estudios no se encontraron diferencias significativas en temperaturas, duración de fototerapia, readmisiones, mortalidad o efectos secundarios; tampoco en los cuestionarios para padres y equipo médico sobre comodidad con los dispositivos.
ConclusionesEstos dos pequeños estudios son un "grano de arena" para la humanización del cuidado neonatal, evitando la separación madre-hijo, tanto para el manejo de la hiperbilirrubinemia intrahospitalaria como para la hiperbilirrubinemia de bajo riesgo en el paciente ambulatorio.
Jaundice due to elevation of indirect bilirubin is frequent in newborns due to an increased red blood cell turnover and liver immaturity. Up to 80 % of preterm newborns develop it, with a predominance of so-called physiological jaundice.1 In term newborns, physiological jaundice manifests between days 2 and 4 post birth with spontaneous resolution after 1–2 weeks, but in the presence of risk factors, it may appear before 36 h post birth with deposition of indirect bilirubin in the basal ganglia of the central nervous system, leading to neuronal apoptosis and neurologic sequelae.2 This risk is greater in preterm infants, as bilirubin may penetrate nervous tissues at lower serum levels due to the immaturity of the blood-brain barrier.3,4
Phototherapy in the blue spectrum (430–490 nm) is the first-line treatment for unconjugated hyperbilirubinaemia, as it causes isomerization of bilirubin to a form that can be eliminated by the kidneys, thus bypassing hepatic conjugation.5 The decision to initiate phototherapy is based on standard curves for chronological age, gestational age, weight and on the presence of risk factors.4,6,7 The effectiveness of phototherapy depends on the spectrum and spectral irradiance of the emitted light.5 Ideally, phototherapy devices emit light covering the largest possible body surface area on the horizontal plane, are durable, generate little heat and can generate optimal wavelengths and irradiance (460–490 nm and ≥30 µW/cm2/nm). The most widely used devices have fluorescent tubes that generate large amounts of heat, thus requiring the use of eye protectors and continuous monitoring of vital signs and body temperature due to increased water losses. Recently developed devices use light-emitting diodes (LED), which achieve a greater decrease in serum bilirubin levels due to a narrower wavelength range, generate minimal heat and require less maintenance.8
Hospital admission for administration of phototherapy is one of the most frequent reasons that mothers and babies need to be separated. Ideally, this separation should be avoided through the use of practical treatment options in order to promote adequate mother-child bonding.9
Novel delivery systems have been developed recently, such as phototherapy blankets and bags that increase the surface area exposed to light and allow parents to feed and hold the baby without interrupting treatment.
Our study aimed to assess the effectiveness and safety of 2 phototherapy devices made with LED fibre optic fabric: 1) a sleeping bag for inpatient treatment (Bilicocoon Bag®) and 2) a large blanket for outpatient treatment that delivers intermittent phototherapy in Kangaroo position for preterm infants with low-risk hyperbilirubinaemia (Bilicocoon Nest®).
Materials and methodsWe conducted 2 clinical randomised controlled trials: one comparing the use of the bag device in hospitalised newborns with birth weights of less than 2000 with hyperbilirubinaemia requiring treatment with conventional phototherapy, and another comparing a large phototherapy blanket with a conventional fibreoptic blanket for preterm newborns with low-risk hyperbilirubinaemia requiring phototherapy and managed in an outpatient Kangaroo mother care (KMC) programme. In both trials, we determined whether the patients required treatment based on the management algorithms published in the guidelines of the American Academy of Pediatrics (AAP) and the National Institute for Health and Care Excellence (NICE).7,10
Sample size calculation and sampling methodPatients were included by consecutive sampling. The patients in the bag trial were newborns admitted to the neonatal care unit (NCU) of the Hospital Universitario San Ignacio (HUSI) of Bogota, Colombia, between March 1, 2016 and October 5, 2017. Patients in the blanket trial were enrolled in the KMC programme of the HUSI between February 8, 2016 and November 15, 2017. In the NCU, the need for phototherapy was established based on serum bilirubin levels, and in the KMC it was established using the Bilicheck® transcutaneous bilirubinometer with subsequent confirmation by measurement of serum bilirubin.
We calculated the necessary sample sizes for 2 groups with a 1:1 allocation ratio for a statistical significant threshold of 0.05 and a power of 80 % for detection of a difference in the mean decrease in bilirubin of at least 0.15 mg/dL/h assuming a standard deviation of 0.2 mg/dL based on the current evidence.11,12 The sample for the phototherapy bag trial was of 29 patients per group, and the sample for the bag trial, adjusted for losses of 5 %, of 32 patients per group.
The exclusion criteria for the bag trial were: patients requiring an incubator (risk of hyperthermia), patients with skin lesions that complicated administration of phototherapy or patients requiring transfer to another hospital. For the blanket trial, the exclusion criteria were onset of jaundice before 48 h post birth, bilirubin level greater than18 mg/dL, diagnosis of diseases requiring hospital admission, extensive skin lesions or parents that could not stay at the KMC programme a minimum of 6 h.
We randomised patients to different groups using computer software and assigned groups to a given intervention using opaque, sealed envelopes.
InterventionAfter obtaining the signed informed consent of the parents, the intervention in the bag trial consisted of conventional phototherapy with blue light emitted by fluorescent tubes or phototherapy with the NeoMedLight Bilicocoon Bag®, with patients in both groups receiving continuous treatment while wearing eye protectors.
In patients with risk factors such as haemolytic disease, glucose 6-phosphate dehydrogenase deficiency, asphyxia, significant lethargy, unstable body temperature, sepsis or acidosis, we measured serum bilirubin levels every 8 h until 2 consecutive values were the same or showed a decrease, followed by measurement of serum bilirubin every 12 h. In patients without risk factors, we measured serum bilirubin levels every 24 h. The treatment was discontinued once the serum bilirubin was below the threshold for indication of phototherapy. In patients with haemolytic disease, we collected blood samples 6–12 h after discontinuation of phototherapy to ensure that bilirubin levels did not increase again.
In the blanket trial, patients received phototherapy with the NeoMedLight Bilicocoon Nest® or the Ohmeda BiliBlanket® in Kangaroo position. The administration of phototherapy was intermittent, given for 2-h intervals with 60 min of rest in between. Levels of bilirubin were measured with the Bilicheck® bilirubinometer and with blood tests at the beginning and end of treatment. If the levels were below the threshold established for treatment or the patient completed three 2-h sessions, a second blood sample was obtained and treatment discontinued, discharging the patient with a follow-up the following day, or the patient was admitted to hospital.
In inpatients, the axillary temperature was measured at start, at 30 min, 1 h, 2 h and thereafter daily and at the end of phototherapy; in outpatients, it was measured at start, at 30 and 60 min and at 2, 4 and 6 h. Patients underwent a physical examination at admission and after completion of treatment to ensure that they did not become dehydrated or develop skin lesions. In the bag trial, patients underwent physical examinations with measurement of weight and testing of fluid and electrolyte balance at baseline, every 24 h and after completion of phototherapy. In both trials, we assessed for the development of side effects.
We surveyed parents and health care staff at the end of treatment to assess the perceived comfort and ease of use of the devices, with items rated on a Likert scale.
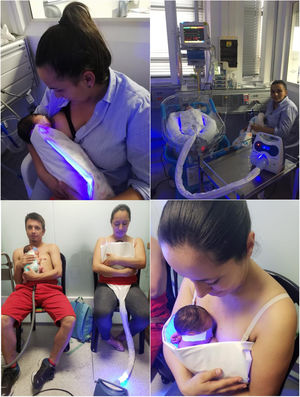
DevicesIn the inpatient trial, we used a phototherapy unit with fluorescent tubes as the source of light (Medix LU-6T®) in the control group and the NeoMedLight Bilicocoon Bag® in the treatment group. The latter is a sleeping bag with ventral and dorsal light exposure, that allows insertion of the infant in a plastic sheath, with two 20 × 30 cm pads of fibre optic fabric connected to a light box, that allows programming the duration of phototherapy. The emission spectrum ranges from 400 to 500 nm with peak wavelengths between 458 and 460 nm. The irradiance is of 35 µW/cm2/nm. The 2 phototherapy pads envelop the baby and are held by a disposable bag that is adjustable to the infant based on weight and height, containing and soothing the infant and preventing the pads from sliding off during use. The bag has flaps on the side that can be opened to allow air circulation, thus preventing hyperthermia.13,14 In the outpatient trial, the device used in the control group was the Ohmeda Medical Biliblanket Plus®, de Ohmeda Medical, which has a high-output light box that delivers light through a fibre optic blanket. The patient is exposed to light in the 400-to-550 nm spectrum. The phototherapy system consists of a light source, a 121.9 cm long fibre optic cable and a lightweight pad measuring 11.12 × 23.83 cm. The light unit houses a high-intensity tungsten halogen lamp with a built-in reflector. In the treatment group, the device used was the NeoMedLight Bilicocoon Nest®, a LED-lit pad with a surface area of 40.5 × 30 cm with a 20 × 30 cm fibre optic fabric swath encased in a protective layer of vinyl with a disposable cover. The emission spectrum is of 400–550 nm, with peak wavelengths between 456 and 460 nm and a mean irradiance of 35 µW/cm2/nm ± 15 %, exceeding the minimum recommended by the AAP for intensive phototherapy13,15 (Fig. 1).
Variables and measurementWe recorded the irradiance of each device at the beginning of the trial, the duration of phototherapy until achievement of a bilirubin level below the threshold for treatment (in hours), the decrease in bilirubin measured in blood samples (in mg/dL/h), axillary body temperatures, the frequency of exchange transfusion in the bag trial, the frequency of hospital admission in the blanket trial, and the mortality. In addition, we recorded the incidence of skin lesions, dehydration or diarrhoea attributable to phototherapy. When it came to the questionnaire on the comfort and ease of use of devices used to survey parents and nursing staff, we compared the frequency of established score categories in the Likert scale for each of the items (1–3 = poor; 4 = neither good nor bad; 5–7 = good).
The trials were not blind; however, most endpoints, such as the level of bilirubin or the body temperature, were not susceptible of being influenced by awareness of assignment status.
Statistical analysisWe compared means for analysis of quantitative data and used the chi square test to analyse qualitative data. We performed a multiple linear regression analysis of the decrease in the level of bilirubin (mg/dL/h) in hospitalised patients. The analysis was by intention to treat.
Both the trial protocols and the informed consent forms were evaluated and approved by the ethics and research committee of the hospital.
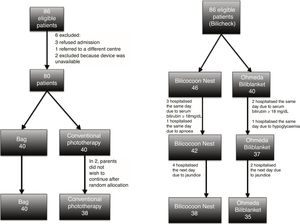
ResultsPhototherapy bagOf 86 eligible patients, 78 completed the study. The initial sample size was 84 for 4 paired samples, however, most patients underwent a maximum of 3 measurements. We recalculated the sample size based on the difference in the mean decrease in the level of bilirubin (mg/dL/h). The new sample size was of 58 patients, 29 per group. We randomised an additional 22 patients (Fig. 2, left).
Phototherapy blanketThe initial sample had 86 patients: 40 assigned to the Ohmeda Biliblanket® and 46 to the NeoMedLight Bilicocoon Nest®. The sample exceeded the calculated size of 64 patients because we initially considered comparison of repeat measurements with the Bilicheck® bilirubinometer, estimating that we needed 94 patients; however, during the study we found a low correlation between the initial and final levels measured with the Bilicheck® and the initial and final serum levels, between 0.43 and 0.53. We decided to assess the decrease in bilirubin levels (mg/dL/h) based on the serum concentration. Of the patients in the initial sample, 7 did not meet the criteria to continue the study, 6 requiring hospital admission the next day due to persistent hyperbilirubinaemia, while phototherapy was effective in 73 (Fig. 2, right).
When it came to the measured irradiance, we found the highest level with the conventional phototherapy device (37.3 ± 10.3 mW/cm2/nm), followed by the bag (36 ± 2.6 mW/cm2/nm) and then the nest (34.8 ± 2.4 mW/cm2/nm). The lowest level corresponded to the Ohmeda Biliblanket® (21.6 ± 8.8 μW/cm2/nm).
Tables 1 and 2 show the characteristics of the patients and the beginning of the trial. When comparing the groups treated with the bag system and conventional phototherapy, we found a greater proportion of patients with early-onset jaundice (<36 h post birth) in the bag group (P = .05). The characteristics of the groups were similar otherwise.
Characteristics of patients admitted to the neonatal unit in the phototherapy bag trial (Bilicocoon Bag®).
| Variable | Conventional phototherapy | Bilicocoon bag® | P |
|---|---|---|---|
| Gestational age at birth, weeks (mean and SD) | 37.3 (1.3) | 37.2 (1.7) | .7a |
| Chronological age at initiation of phototherapy, hours (median and interquartile range) | 48 (24–144) | 36 (27–59) | .2a |
| Weight at initiation of phototherapy, grams (median and interquartile range) | 2560 (2235–2940) | 2593 (2230–3048) | .1a |
| Initial temperature, °C (median and interquartile range) | 36.7 (36.6–36.8) | 36.7 (36.5–36.8) | .4a |
| Initial serum bilirubin, mg/dL (mean and SD) | 12.8 (4.2) | 11.6 (3.8) | .2a |
| Sex, n (%) | .1b | ||
| Male | 25 (65.8 %) | 19 (47.5 %) | |
| Female | 13 (34.2 %) | 21 (52.5 %) | |
| Race | 100 % mixed race | 100 % mixed race | — |
| Haemolytic disease, n (%) | 9 (23.7 %) | 5 (12.8 %) | .2b |
| Early-onset jaundice (< 36 h), n (%) | 10 (26.3 %) | 19 (47.5 %) | .05b |
Characteristics of patients managed in the outpatient kangaroo care programme in the phototherapy blanket trial (Bilicocoon Nest®).
| Variable | Ohmeda Biliblanket® | Bilicocoon Nest® | P |
|---|---|---|---|
| Gestational age, weeks (median and interquartile range) | 36.1 (35.3–36.5) | 35.8 (35.2–37.0) | .9a |
| Chronological age at initiation of phototherapy, hours (mean and SD) | 150 (65) | 159 (76) | .5a |
| Weight at admission, grams (median and interquartile range) | 2195 (2105–2310) | 2175 (2060–2240) | .2a |
| Initial temperature, °C (median and interquartile range) | 36.8 (36.6–37.0) | 36.6 (36.6–36.9) | .7a |
| Initial bilirubin, mg/dL (mean and SD) | 15.3 (2.4) | 15.0 (2.8) | .7a |
| Sex, n (%) | .8b | ||
| Male | 20 (50 %) | 16 (47 %) | |
| Female | 20 (50 %) | 18 (53 %) | |
| Race, n (%) | .5b | ||
| Mixed race | 38 (95 %) | 34 (100 %) | |
| Black | 2 (5 %) | 0 |
As for the outcomes, we found a similar decrease in bilirubin levels with the bag and the conventional phototherapy device (difference in means, 0.03 mg/dL/h; 95 % CI, −0.04 to 0.09). We found difference in body temperature at the end of treatment that was clinically insignificant. There were no cases of hyperthermia, adverse events, need of exchange transfusion or death (Table 3).
Outcomes. Phototherapy bag (Bilicocoon Bag®) - patients hospitalised in the neonatal unit.
| Variable | Conventional phototherapy | Phototherapy bag | P |
|---|---|---|---|
| Final temperature, °C (median and interquartile range) | 36.7 (36.6–36.8) | 36.9 (36.7–37.0) | .01a |
| Hours of phototherapy (median and interquartile range) | 24 (20–30) | 27.5(18–40) | .3a |
| Bilirubin decrease rate (mean and SD) | 0.10 (0.05–0.15) | 0.07 (0.02–0.12) | .4b |
| Bilirubin decrease rate in patients with early-onset jaundice (≥ 36 h) (mean and SD) | −0.03 (0.13) | −0.02 (0.06) | .8b |
| Bilirubin decrease rate in patients without early-onset jaundice (mean and SD) | 0.14 (0.13) | 0.15 (0.14) | .9b |
The decrease in bilirubin levels with the new phototherapy blanket (Bilicocoon Nest®) was significantly greater compared to the one achieved by the Ohmeda Biliblanket®. We did not find differences in final body temperature, hours of phototherapy, days of phototherapy, readmission rate, side effects or mortality (Table 4).
Outcomes. Phototherapy blanket (Bilicocoon Nest®) – Outpatient kangaroo care programme.
| Variable | Bilicocoon Blanket® | Biliblanket de Ohmeda® | P |
|---|---|---|---|
| Final temperature, °C (median and interquartile range) | 37.20 (37.1–37.3) | 37.25 (37.1–37.3) | .4a |
| Hours of phototherapy (median and interquartile range) | 4 (4–6) | 4 (4–6) | .6a |
| Days of phototherapy (mean and SD) | 2.2 (0.9) | 2.4 (1.3) | .5b |
| Length of inpatient stay in days | 2.0 (5) | 4.0 (12) | .9c |
| Exanthema | 1.0 (2.5) | 0 | .9c |
| Serum bilirubin decrease rate (mg/dL/h) (mean and SD) | 0.02 (0.04) | 0.2 (0.4) | .0002b |
The linear regression model in which the bilirubin decrease rate was the dependent variable and the type of device and the presence of early-onset jaundice as predictors, found that the bilirubin decrease rate was not associated with the phototherapy device used (P = 0.8) after controlling for the effect of early-onset jaundice (< 36 h) (Table 5).
Linear regression analysis. Bilirubin decrease rate – Phototherapy bag (Bilicocoon Bag®) – patients hospitalised in neonatal unit.
| Bilirubin decrease rate (mg/dL/h) | Coefficient | Standard error | P | 95 % CI |
|---|---|---|---|---|
| Device | 0.007 | 0.029 | .8 | −0.052 to 0.065 |
| Early-onset jaundice (<36 h) | −0.171 | 0.030 | < .001 | −0.232 to −0.110 |
| Constant | 0.142 | 0.022 | < .001 | 0.098 to 0.186 |
Observations = 70.
F (2.67) = 16.3.
p >F ≤ 0.001.
R2 = 0.33.
Adjusted R2 = 0.31.
In the inpatient trial, the ratings on the Likert scale given by parents and nurses in the satisfaction survey for the bag system showed that parents did not perceive any differences in comfort when it came to kangaroo care, breastfeeding, body temperature or exposure to light. Nurses expressed that infants seemed to be less comfortable in the bag system (Table 6).
Satisfaction of nurses with the Bilicocoon Bag® - patients hospitalised in neonatal unit.
| Variable | Conventional phototherapy | Phototherapy bag | Pa | ||
|---|---|---|---|---|---|
| Ease to place infant in device | Poor | 0 | Poor | 1 | .06 |
| Neither good nor bad | 0 | Neither good nor bad | 0 | ||
| Good | 34 | Good | 36 | ||
| Comfort during phototherapy | Poor | 1 | Poor | 1 | .25 |
| Neither good nor bad | 0 | Neither good nor bad | 3 | ||
| Good | 33 | Good | 33 | ||
| Ease of breastfeeding | Poor | 2 | Poor | 1 | .12 |
| Neither good nor bad | 0 | Neither good nor bad | 3 | ||
| Good | 32 | Good | 33 | ||
| Tolerability of emitted light | Poor | 4 | Poor | 0 | .19 |
| Neither good nor bad | 1 | Neither good nor bad | 1 | ||
| Good | 29 | Good | 36 | ||
| Tolerability of heat emitted by device | Poor | 2 | Poor | 1 | .55 |
| Neither good nor bad | 4 | Neither good nor bad | 2 | ||
| Good | 28 | Good | 34 | ||
| Perceived comfort of materials | Poor | 1 | Poor | 1 | .26 |
| Neither good nor bad | 1 | Neither good nor bad | 5 | ||
| Good | 32 | Good | 31 | ||
| Ease of cleaning | Poor | 2 | Poor | 1 | .64 |
| Neither good nor bad | 2 | Neither good nor bad | 2 | ||
| Good | 30 | Good | 34 | ||
| Perceived weight | Poor | 4 | Poor | 5 | .52 |
| Neither good nor bad | 4 | Neither good nor bad | 5 | ||
| Good | 26 | Good | 27 | ||
| Ease of disconnecting | Poor | 1 | Poor | 1 | .47 |
| Neither good nor bad | 1 | Neither good nor bad | 0 | ||
| Good | 32 | Good | 36 | ||
| Ease of removing baby | Poor | 0 | Poor | 0 | .36 |
| Neither good nor bad | 1 | Neither good nor bad | 0 | ||
| Good | 33 | Good | 37 | ||
| Perceived comfort of baby | Poor | 0 | Poor | 2 | .01 |
| Neither good nor bad | 2 | Neither good nor bad | 2 | ||
| Good | 32 | Good | 33 | ||
In the blanket trial, the survey showed that parents and nurses did not perceive any differences between the compared devices.
DiscussionHyperbilirubinaemia is the leading cause of hospitalization in newborns. In 1985, Brown et al. proved that early phototherapy (given within 24 ± 12 h from birth) prevents hyperbilirubinaemia in newborns with weights of less than 2000 g, including those with neonatal haemolysis, with a reduction in the proportion requiring exchange transfusion from 23.9 % to 4.8 %.16 In recent years, strategies such as universal screening for haemolytic disease due to Rh incompatibility, coordination of perinatal and neonatal care and the effective use of phototherapy based on hourly levels of total serum bilirubin and the Buthani nomogram17 have virtually eliminated the risk of kernicterus in term newborns. However, when it comes to infants born before 35 weeks’ gestation, no studies have established the bilirubin level threshold for indication of treatment at each hour after birth, the nomograms used for treatment have been obtained by extrapolation of those available for term newborns,18 and relatively low serum levels of bilirubin can result in neurodevelopmental abnormalities. As a result, some infants that need treatment do not receive it, while others with physiological jaundice receive superfluous treatment. This highlights the need for appropriate, effective and individualised treatment for risk reduction in preterm infants. In addition, the separation from the mother that results from hospitalization for the purpose of administering phototherapy causes attachment and breastfeeding problems and exposes the infant to infection, which has led some authors to propose the possibility of treating babies with low-risk hyperbilirubinaemia requiring intervention with intermittent phototherapy at the outpatient level.9
New phototherapy devices developed with light-emitting diode (LED) technology, deliver light in a narrower spectrum (450−475 nm) that optimises the isomerization of bilirubin. The use of LED light sources is now combined with fibre optic fabric that allows the administration of phototherapy in direct contact with the patient’s skin, like a blanket, which allows mothers to hold and breastfeed their babies without interrupting inpatient treatment or during administration of intermittent phototherapy in outpatient follow-up programmes.
In this study, we tried 2 devices: a bag system for inpatient treatment and a large blanket for intermittent outpatient phototherapy.
The results showed that the bag system achieved a bilirubin concentration decrease rate that was at least similar to the one achieved with conventional phototherapy. We found body temperatures that were slightly higher in patients treated with the bag system, but this increase was not clinically relevant. By chance, there was a higher proportion of patients with early-onset jaundice (onset before 36 h post birth) in the bag system group compared to the conventional phototherapy group (19 vs 10, P = .05). We decided to take this variable into account thinking of those patients without a diagnosis of haemolytic disease but who had a degree of jaundice that was considered pathological. We performed a multivariate linear regression analysis controlling for the presence of early-onset jaundice and found no statistically significant differences in the bilirubin decrease rate based on the type of device used. We found that early-onset jaundice had a negative impact on the bilirubin decrease rate, probably because these cases mostly corresponded to high-risk jaundice cases. We did not observe any adverse effects of phototherapy, such as rash, dehydration or diarrhoea.
The survey of parents and nurses revealed no differences in most aspects concerning the perceived comfort of the bag system versus fluorescent tube units. The only relevant finding was that nurses considered that the baby was less comfortable with the bag system (P = .01); this could be due to the resistance the staff may feel toward a new device that requires training for its use, and should be evaluated with a larger survey. The fact that parents of hospitalised infants did not perceive additional benefits with the bag system when it came to holding or nursing the baby, which seem obvious advantages, could be due to the fact that the neonatal unit where the trial was conducted is open to parents around the clock, allowing them to stay with their babies for long periods of time and breastfeeding on demand, which may have contributed to parents not perceiving a difference.
When it came to the outpatient management of jaundice in the follow-up KMC programme, the fact that hospitalization due to jaundice is always traumatic in these families in which parents are barely starting to adapt to their roles as main caregivers and the need to keep the baby in skin-to-skin contact 24 h a day at home, led to the introduction years ago of outpatient intermittent phototherapy using a fibre optic blanket attached to a small LED light source, the Ohmeda Biliblanket®. In our study, we sought to compare this device with a larger blanket covering the whole body of the infant without needing to interrupt kangaroo care, the Bilicocoon Nest®. The decrease in bilirubin levels was clearly superior with the latter: 0.2 (0.4) mg/dL/h vs 0.02 (0.04) mg/dL/h (P = .0002), probably due to the larger size and greater irradiance of this new device. We did not find differences in temperature, duration of phototherapy, hospital readmission, exanthema, dehydration or mortality. We also found no differences in the perceived comfort of use of the devices in either parents or nurses, which may be due to both devices being compatible with kangaroo care and their only discernible difference being the size of the blanket, which does not have a big impact on comfort or ease of use.
The development of new phototherapy devices as effective as those of conventional phototherapy while allowing increased parent-infant contact to facilitate bonding and breastfeeding is essential for the humane treatment of jaundice at the inpatient or outpatient level.
FundingWe received support from the Fundación Canguro (Bogota, Colombia) and the Department of Paediatrics of the Pontificia Universidad Javeriana (Bogota, Colombia) to cover the cost of office supplies and the wages corresponding to the time researchers allocated to the project.
Conflicts of interestThe French company Neomedlight donated the Bilicocoon Bag and the Bilicocoon Nest to the Fundación Canguro. The authors have no ties to this company.
We thank the physicians and nurses of the Hospital Universitario San Ignacio and the Department of Paediatrics of the Pontificia Universidad Javeriana for their collaboration in the study. We also thank the parents of our patients.
Please cite this article as: Montealegre A, Charpak N, Parra A, Devia C, Coca I, Bertolotto AM. Efectividad y seguridad de 2 dispositivos de fototerapia para el manejo humanizado de la ictericia. An Pediatr (Barc). 2020;92:79–87.