Ovarian tumours are rare in childhood, and account for 1–5% of all tumours. The aim of this study is to determine the epidemiological features, histological subtypes, and therapeutic management of ovarian solid ovarian tumours of the paediatric population of the province of Cordoba, in Spain.
Material and methodsA retrospective, descriptive, observational and institutional study was conducted in which a review was made of the clinical histories of patients younger than 14 years-old diagnosed with ovarian tumours, excluding secondary tumours in a University Hospital between 1994 and 2017. A review was carried out on the age, clinical presentation, laterality, diagnostic methodology, treatment, histopathology, and evolution of these tumours.
ResultsA total of 37 ovarian tumours were reviewed in 31 patients, 6 of them being bilateral. The mean age was 10.3 (0–14) years, with 58% presenting as a palpable mass. There was no predominance of laterality. The tumour markers were negative. Conservative surgery was performed in 29.7% and adnexectomy in 70.3%. Only one case required post-operative adjuvant chemotherapy treatment (stage I immature teratoma with peritoneal gliomatosis). The histological study shows a predominance of germ cell tumours (65%) against those of epithelial lineage (22%). There were 3 stromal tumours that corresponded to fibroma (Gorlin syndrome), and bilateral gonadoblastoma associated with Frasier syndrome. The most frequent type of tumour was mature cystic teratoma (35.1%). There were no complications in the follow-up.
ConclusionsGiven that most childhood ovarian tumours are benign, conservative surgery is considered as the first choice, being even more important in bilateral tumours. If there is a family history, it is essential to carry out molecular genetic studies, to rule out associated syndromes.
Los tumores ováricos son raros en la infancia y representan entre el 1 y el 5% de todos los tumores sólidos. Nuestro objetivo es conocer las características epidemiológicas, los subtipos histológicos y el manejo terapéutico de los tumores sólidos ováricos de la población pediátrica de la provincia de Córdoba.
Material y métodosSe realizó un estudio retrospectivo, descriptivo, observacional, en el que se han revisado las historias clínicas de pacientes ≤14años diagnosticadas de tumores sólidos ováricos en un hospital de tercer nivel entre los años 1994 y 2017, excluyéndose los tumores secundarios. Se revisó la edad, la presentación clínica, la lateralidad, la metodología diagnóstica, el tratamiento, la anatomía patológica y la evolución.
ResultadosSe revisaron 37 tumores ováricos en 31 pacientes, siendo 6 bilaterales. La edad media fue de 10,3años (0-14). El 58% debutaron como masa palpable. No existe predominio de lateralidad. Los marcadores tumorales fueron negativos. Se practicó cirugía conservadora en el 29,7% y anexectomía en el 70,3%. Solo un teratoma inmaduro estadioI con gliomatosis peritoneal precisó tratamiento quimioterápico adyuvante postoperatorio. El estudio histológico demuestra un predominio de tumores de células germinales (65%) frente a los de estirpe epitelial (22%). Destacan 3 tumores estromales que corresponden a fibromas (síndrome de Gorlin) y un gonadoblastoma bilateral asociado a síndrome de Frasier. El tipo de tumor más frecuente fue el teratoma quístico maduro (35,1%). Evolución favorable en todos los casos.
ConclusionesDada la alta tasa de benignidad de los tumores ováricos en la infancia, la cirugía conservadora debe ser de primera elección, sobre todo en los bilaterales. Si existen antecedentes hereditarios, es imprescindible realizar estudios genéticos moleculares para descartar síndromes asociados.
Ovarian tumours are rare in the paediatric age group and amount to 1–5% of childhood tumours, with an estimated annual incidence of 2.6 cases per 100000 individuals.1,2 They are most frequent between ages 10 and 14 years, with a higher prevalence of malignant tumours in older girls. The age distribution in the paediatric population is bimodal, with peaks at 2–3 years and 12–15 years. The incidence peaks in adolescence, when 50% of cases are diagnosed, and they are extremely rare before age 1 year.3
They are classified into 3 groups based on the scheme proposed by the World Health Organization4–6: surface epithelial-stromal tumours, germ cell tumours and sex cord-stromal tumours. In the paediatric age group, germ cell tumours amount to 90% of all ovarian tumours, and the most frequent histological types are mature cystic teratoma or dermoid cyst.7
Excluding benign cystic tumours, the most frequent solid tumours in the reproductive system of girls and female adolescents are germ cell tumours, which amount to 90% of the total, contrary to the adult female population, in which epithelial tumours predominate, amounting to 75% of the total (of which 80% are carcinomas) while germ cell tumours amount to 15–25% of the total.
The clinical presentation of ovarian tumours is highly nonspecific,8 and the most frequent manifestations are abdominal pain, abdominal distension and presence of a palpable mass. The abdominal pain usually has an insidious onset and is associated with abdominal distension, but it can have an acute onset due to torsion, rupture or bleeding, presenting as an acute surgical abdomen.
As is the case in the adult population, the diagnosis is based on the findings of imaging tests, tests for tumour markers and the pathological examination.
Ultrasound examination9 helps locate the mass and define its characteristics. Malignant tumours tend to have irregular borders and necrotic tissue in the middle of the mass and appear as poorly defined soft tissue masses.10 In contrast, benign tumours are hypoechoic and may feature posterior enhancement. Ovarian cysts are generally anechoic and thin walled.
To date, computed tomography (CT) is considered the gold standard for the staging of ovarian cancer before surgery. Magnetic resonance imaging (MRI) is reserved for cases where there is uncertainty about the primary origin of the mass due to its large size.
While testing for tumour markers11 cannot confirm the benign or malignant nature of the tumour (as 20% of positive results correspond to benign tumours), it can be very helpful to guide the approach to treatment and the subsequent follow-up and to assess the response to treatment. The markers used most commonly are alpha-foetoprotein (AFP), beta-human chorionic gonadotropin (bHCG) and lactate dehydrogenase (LDH).
The diagnosis is confirmed by the pathological examination of surgical samples or samples obtained by ultrasound-guided biopsy.
In paediatric practice, malignant ovarian tumours are a minority and their clinical presentation is nonspecific. Therefore, it is important to ensure that they do not go unnoticed and that they are diagnosed early in order to deliver appropriate treatment. The treatment should be selected based on the type of tumour and its extension.
Surgery plays a key role in the treatment of ovarian tumours and can achieve excision of the entire mass in more than 90% of cases. It is essential to adhere to standards in surgical oncology, performing a salpingo-oophorectomy via laparotomy or through a laparoscopic approach. If the preoperative assessment cannot not establish the resectability of the malignant tumour, an ultrasound-guided biopsy should be performed and, and the surgery to remove the tumour programmed once neoadjuvant chemotherapy has been completed.12
Historically, laparoscopic surgery for treatment of lesions known or strongly suspected to be malignant was discouraged. At present, with the development of minimally invasive surgery, this position is no longer valid, and the approach is selected based on the characteristics of the mass. In case of benign and bilateral tumours, the optimal approach is conservative surgery with the aim of preserving the least damaged region and the long-term fertility of the patient.12
The specific aim of the study was to analyse ovarian tumours in the paediatric population of the province of Cordoba, Spain.
Material and methodsWe conducted a retrospective, observational and descriptive study by reviewing the health records of girls aged 14 years or less with a diagnosis of solid ovarian tumour operated in the Department of Paediatric Surgery of the Hospital Universitario Reina Sofía in Cordoba, Spain, between 1994 and 2017. We excluded benign cystic lesions and metastatic tumours.
In this study, we analysed the age at onset, clinical presentation, imaging features, tumour markers, surgical approach, pathological findings and outcomes of paediatric patients with ovarian cancer. The variables under study were the following:
- -
Age. Quantitative.
- -
Clinical presentation. Qualitative.
- -
Ultrasound/CT/MRI. Dichotomous qualitative Yes/No. In all cases, an ultrasound scan was made, followed by a CT scan and in some cases MRI.
- -
Tumour markers. Dichotomous qualitative Positive/Negative.
- -
Location. Dichotomous qualitative Right/Left.
- -
Laterality. Dichotomous qualitative Bilateral/Unilateral.
- -
Surgical treatment. Qualitative (open or laparoscopic). It is the key tool for diagnosis.
- -
Biopsy. Dichotomous qualitative Yes/No. Performed in case of suspected involvement of the contralateral ovary or another abdominopelvic organ and for lymph node sampling.
- -
Cytological analysis of peritoneal fluid. Dichotomous qualitative Yes/No. Performed in every patient following the standards of surgical oncology.
- -
Adjuvant treatment. Dichotomous qualitative Yes/No.
- -
Anatomical pathology. Qualitative.
- -
Complications. Dichotomous qualitative Yes/No.
- -
Mortality. Dichotomous qualitative Yes/No.
We collected data in an Excel spreadsheet designed for the purpose and subsequently exported them to the software SPSS version 17 to perform the descriptive analysis. We have summarised continuous variables as mean±standard deviation (SD) and categorical variables as absolute or relative frequencies with the corresponding 95% confidence intervals (CIs).
ResultsWe reviewed 37 ovarian tumours in 31 patients, 6 of them bilateral. We divided the sample into 3 age groups: 0–5 years (18.9%), 6–10 years (18.9%) and 11–14 years (62.2%), which was the largest group with a total de 23 patients. The mean age was 10.32±4.27 years (range, 0–14).
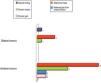
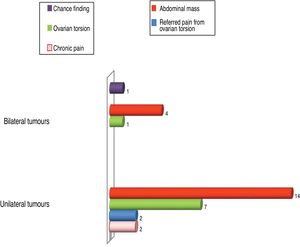
The most frequent presenting feature was palpable abdominal mass (n=18), found in 48.6% of patients, followed by acute ovarian torsion in 8 (21.6%). Chronic pain was reported by 6.5% patients (n=2), while another 6.5% (n=2) had onset with referred pain caused by ovarian torsion. In 1 patient the tumour was a chance finding (Fig. 1). There was no predominant side: the right ovary was involved in 51.4% of patients and the left in 48.6%. Of the 31 patients in the sample, 32.4% had bilateral tumours, while 67.6% had unilateral tumours.
When it came to imaging tests, the first test performed in all patients was the ultrasound examination, used to differentiate cystic from solid masses, followed by CT, used to rule out metastasis and for staging. Magnetic resonance imaging was used if the primary origin of the mass was uncertain and to assess the relationship of the mass with adjacent structures.
All patients underwent blood tests for detection of tumour markers, with values within the normal range: LDH (normal range: 125–220mU/mL); AFP (normal range: 1.10–8.10ng/mL) and bHCG (normal range: 1.20–5.00mU/mL).
The most frequent surgical intervention was adnexectomy (salpingo-oophorectomy), performed for resection of 26 tumours (70.3%), followed by removal of the tumour via laparotomy (16.2%) and removal of the tumour with laparoscopic surgery (5.4%). In all cases, the treatment approach adhered to the care standards of surgical oncology. Only 1 patient with a bilateral tumour (2.7%) underwent a biopsy of the contralateral ovary as the sole intervention in that side.
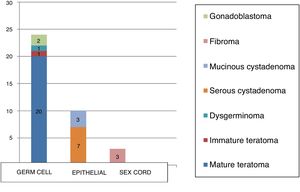
The diagnosis was confirmed by histological examination, with a predominance of germ cell tumours (65%) over epithelial tumours (22%). There were 3 sex cord tumours, 2 of them fibromas (1 unilateral, 1 in a patient with Gorlin syndrome) and 1 mixed tumour (bilateral gonadoblastoma in a patient with Frasier syndrome).
The most frequent types of tumour were mature cystic teratoma (54.1%) and serous cystadenomas (18.9%), and there were 3 cases of mucinous cystadenoma (8.1%), 3 of ovarian fibroma (8.1%), 2 of gonadoblastoma (5.4%), 1 of immature cystic teratoma (2.7%) and 1 of dysgerminoma (2.7%) (Table 1).
Solid ovarian tumours by histological features and laterality.
| Histology | Unilateral ovarian tumours (%) | Bilateral ovarian tumours (%) | |
|---|---|---|---|
| n=31 | n=6 | ||
| Right | Left | ||
| Benign teratoma (mature cystic) | 13.50% | 21.61% | 21.61% |
| Malignant teratoma (immature) | 2.70% | ||
| Dysgerminoma | 2.70% | ||
| Gonadoblastoma | 2.70% | ||
| Serous cystadenoma | 13.5% | 5.40% | |
| Mucinous cystadenoma | 5.40% | 2.70% | |
| Fibroma | 2.70% | 5.40% | |
| Total | 35.1% | 32.41% | 32.4% |
Out of the 6 bilateral tumours, 2 corresponded to girls with mature teratoma and ovarian torsion, who underwent an initial adnexectomy followed by laparoscopic tumourectomy of an additional metachronous mature teratoma. Another bilateral case corresponded to a girl with a large immature teratoma with gliomatosis peritonei that later developed a contralateral mature teratoma, treated with fertility-sparing surgery.
We ought to highlight the case of a girl aged 12 years with a bilateral ovarian tumour that received a diagnosis of Gorlin syndrome,13 later confirmed by the detection of a mutation in the PTCH1 gene in chromosome 9. This is a rare autosomal dominant disease characterised by the development of odontogenic cysts, basal cell naevi and skeletal abnormalities. It may be associated with ovarian fibromas, which are typically bilateral in the context of this syndrome. The patient had a personal history of congenital hydrocephalus and a family history of ovarian adenocarcinoma in a maternal aunt. The patient underwent a left salpingo-oophorectomy and complete gross resection of the tumour in the right side with regional lymphadenectomy. Given the presence of a bilateral fibroma, highly suggestive of Gorlin syndrome, the subsequent evaluation included not only genetic testing but also a panoramic radiograph, which revealed the presence of odontogenic keratocysts, and cranial radiographs that revealed calcification of the falx cerebri.
Another patient aged 5 years had Frasier syndrome14 associated with bilateral gonadoblastoma, which was detected during an exploratory laparoscopy and treated with a bilateral adnexectomy. Frasier syndrome is a rare disorder caused by a mutation in the WT1 gene (a gene that suppresses Wilms tumour) and characterised by male pseudohermaphroditism (external genitalia with female appearance despite XY genotype), renal disease and a high risk of developing Wilms tumour.
When it came to the postoperative treatment, the only patient that required adjuvant chemotherapy was the one with stage I immature teratoma and gliomatosis peritonei due to the risk of rapid growth and spread of this histological type,15–18 delivered following the MAKEI 96 protocol with 4 cycles of cisplatin, etoposide and ifosfamide (PEI).
There were few postoperative complications, the most salient of which was an abdominal wall haematoma. The overall survival in this case series is 100% (at 12 months to 22 years of follow-up).
DiscussionOvarian tumours manifest with heterogeneous and nonspecific signs and symptoms. While in adult patients the most frequent manifestation is a change in menstruation, the presentation is even more nonspecific in the paediatric population (vomiting or fever). In our study, the most frequent clinical presentation was presence of a palpable abdominal mass, while in the previous literature the frequency of abdominal pain as the initial symptom is somewhat higher compared to the palpable mass. Therefore,19 ultrasound examination is a key tool to establish the aetiology of acute or chronic abdominal pain or a palpable abdominal mass.
As for tumour markers, most case series in the literature report elevation of AFP and bHCG20; but in our series, since most tumours were benign, both were negative in all patients. These markers are useful both for diagnosis and to assess response to treatment and patient outcomes.
The staging of primary ovarian tumours was based on the classification of the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) and the classification proposed by the Children's Oncology Group (COG). Both are based on the findings of imaging tests and the examination of surgical specimens.21
As for the management of benign ovarian neoplasms, more conservative treatment is recommended at present,22,23 avoiding performance of salpingo-oophorectomy whenever possible. However, a high proportion of patients in our study underwent adnexectomy and a low proportion laparoscopic surgery,24 mainly due to the large size of the tumours and the risk of seeding due to tumour rupture; in these cases, the surgical approach was infraumbilical laparotomy with a Pfannenstiel incision. Furthermore, many cases in the sample predate the use of conservative surgery with a laparoscopic approach.
On the other hand, a more aggressive approach was adopted in patients with acute ovarian torsion or referred pain from ovarian torsion in whom malignancy was suspected or the viability of the ovarian parenchyma uncertain after detorsion. However, at present the most common approach is minimally invasive surgery with preservation of ovarian parenchyma.
In our sample, 65% of cases corresponded to germ cell tumours compared to 22% for epithelial tumours, and only 3 were sex cord tumours (8.1%; all of them fibromas). Two patients (5.4%) had mixed histology: serous cystadenoma associated with benign cystic teratoma.
The results of our study (Fig. 2) were consistent with those of similar studies, although the percentage of surface epithelial tumours (22%) was higher compared to the previous literature (percentages <15%), although we also found studies where these tumours amounted to up to 30% of the total, nearly double the figure reported in most other studies.25–27
Some of the studies in the reviewed literature suggest that tumour size is an important predictor of malignancy26,27; however, in our experience, with the exception of the immature teratoma, which was of giant size, the rest of ovarian tumours, although substantially large (diameter >10cm), were benign.
ConclusionsIn this case series, the most frequent type of paediatric solid ovarian tumour were germ cell tumours, with a predominance of mature teratomas. In addition, we found a higher proportion of epithelial tumours (22%) than previously described in the literature.
Conserving surgery, whether open or laparoscopic, should be the first-line treatment, and is even more important in bilateral tumours with the aim of preserving future endocrine function and fertility.
In case of a relevant family history, it is essential to perform molecular genetic tests to rule out associated syndromes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Vázquez Rueda F, Murcia Pascual FJ, Siu Uribe A, Ortega Salas RM, Escassi Gil Á, Garrido Pérez JI, et al. Análisis de los tumores sólidos ováricos pediátricos en nuestra población. An Pediatr (Barc). 2020;92:88–93.
Previous presentation: partial results of this study were presented as an electronic poster at the LV Congress of the Sociedad Española de Cirugía Pediátrica; May 26–27, 2016; Oviedo, Spain.