The fortification of maternal milk (MM) is a standard practice in order to achieve the requirements needed for the growth and development of the premature newborn. However, its osmolality could increase. According to the American Paediatrics Academy, it is recommended not to exceed 450mOsm/kg (approximately 400mOsm/L) in the diet of the infant, even though the safety limit is estimated to be between 400 and 600mOsm/kg.
The aim of this study is to determine the osmolality of thawed and fortified donated MM (DMM).
MethodAn analysis was performed on DMM of 6 healthy mothers, without fortifying, and with 4 levels of fortification. Measurement of the samples was carried out in triplicate at 0, 4, 9, and 24h after their preparation. They were stored refrigerated (2–8°C) between measurements. The study groups were: (A) non-fortified DMM; (B) DMM with vitamins added; (C) with the addition of a fortifier; (D) with the addition of a low-dose protein formula; and (E) with the addition of a high-dose protein formula. The osmolality determinations were carried out using a freezing-point osmometer. The data analysis was performed using R software (v.3.5.1).
ResultsA total of 30 samples were analysed with 360 measurements. The osmolality of the DMM at t=0h was 301mOsm/kg (SD 5.2) and slightly increased with time to 308.11mOsm/kg (SD 5.21) after 24h (t=24h), being maintained within the safety limits. The addition of vitamins (Group B) did not significantly increase the osmolality. The addition of a fortifier (C) and a low dose (D) or high dose (E) protein formula produced an increase in the baseline osmolality that increased statistically significantly in time (P=.007), but with no differences between the C, D, and E types. There were differences between the osmolality at t=0 with the fortification according to the manufacturer's data sheet (339mOsm/L) and the findings in our laboratory (432.33mOsm/L).
ConclusionThe osmolality values found in the thawed DMM samples were similar to those of other studies. The fortification of the DMM samples and their storage refrigerated at 2–8°C for 24h increased the osmolality, but keeping them within the safety limits.
La fortificación de la leche materna (LM) es una práctica habitual para alcanzar los aportes necesarios para el crecimiento y desarrollo del recién nacido prematuro. Sin embargo, puede aumentar su osmolalidad. Según la Academia Americana de Pediatría, se recomienda no superar 450 mOsm/kg (aproximadamente 400 mOsm/l) en la alimentación del lactante, aunque el límite de la seguridad se estima entre 400-600 mOsm/kg.
El objetivo de este estudio es determinar la osmolalidad de la LM donada (LMD) descongelada y fortificada.
MétodoSe analizó la LMD de 6 madres sanas, sin fortificar y con 4 pautas de fortificación, realizando mediciones por triplicado de las muestras a tiempo 0, 4, 9 y 24 h tras su preparación. Entre mediciones se almacenó refrigerada (2-8°C). Los grupos de estudio fueron: 1) A: LMD no fortificada; 2) B: LMD con adición de vitaminas; 3) C: con adición de fortificante; 4) D: con adición de módulo proteico a dosis baja, y 5) E: con adición de módulo proteico a dosis alta. Las determinaciones de osmolalidad se realizaron un osmómetro crioscópico. El análisis de datos se realizó con R software (v.3.5.1).
ResultadosSe analizaron un total de 30 muestras (360 mediciones). La osmolalidad de la LMD a t=0h fue 301 mOsm/kg (DE 5,2) y aumentó ligeramente con el tiempo a 308,11 mOsm/kg (DE 5,21) después de 24 h (t=24h), manteniéndose en los límites de seguridad. La adición de vitaminas no aumentó la osmolalidad de manera significativa (grupo B). La adición de fortificante (C) y módulo de proteínas con dosis baja (D) o dosis alta (E) produjo un aumento de osmolalidad basal que se incrementó en el tiempo de manera estadísticamente significativa (p=0,007) pero sin diferencias entre los tipos C, D y E. Hubo diferencias entre la osmolalidad en t=0 con la fortificación según la ficha técnica del fabricante (339 mOsm/l) y los hallazgos en nuestro laboratorio (432,33 mOsm/l).
ConclusiónLos valores de osmolalidad hallados en las muestras de LMD descongelada son similares a los de otros estudios. La fortificación de la LMD y su conservación refrigerada entre 2-8°C durante 24h aumenta la osmolalidad, pero manteniéndose dentro de los límites de seguridad.
Breastfeeding (BF) is the gold standard for nutrition of preterm (PT) infants due to its short- and long-term health benefits. If the mother's own milk is not available, banked donor human milk (DHM) is considered the best alternative.1 However, in many instances DHM alone does not meet the full nutritional requirements of the infant.1–3 To optimise nutrient intake in this group to improve growth and development outcomes, fortifiers have been developed to add to human milk to contribute energy, protein, minerals and vitamins. Most fortifiers are multicomponent preparations derived from bovine milk, although there are also fortifiers made from DHM and single-component fortifiers in the market.4 These products are widely used in neonatal units, with a standardised approach or with an individualised approach whereby the addition of fortifiers is adjusted based on the analysis of milk composition or blood urea nitrogen (BUN) levels. Early studies comparing this nutritional management in PT infants with conventional BF found improvement in anthropometric measures, nitrogen balance and BUN levels, serum albumin, total protein and prealbumin levels, and normalisation of bone markers.5,6 However, a recent review only found a difference in anthropometric outcomes during the hospital stay, but no other relevant benefits, complications or adverse effects.5 Nevertheless, fortification of human milk is not free of drawbacks, as processing the milk results in a decreased absorption of fats and an increase in milk osmolality. Osmolarity and osmolality are terms used to express the total concentration of solutes, or osmols, of a solution. Broadly speaking, osmolality measures concentration based on the osmols of solute per kilogram of solvent, expressed as mOsm/kg, while osmolarity refers to the osmols of solute per litre of solvent, expressed as mOsm/L. Since the volume of the solution changes with the addition of solute and with changes in temperature and pressure, the osmolarity is difficult to measure. In contrast, the mass of solvent remains constant regardless of changes in temperature or pressure, so osmolality is easier to evaluate and therefore used more widely, and it is currently considered the standard measure of concentration.7
The association between a high osmolality in the nutritional intake and the risk of gastrointestinal problems and necrotising enterocolitis (NEC) is a source of concern.7 To reduce this risk, the American Academy of Pediatrics (AAP) recommends a maximum threshold of 450mOsm/kg (approximately 400mOsm/L)8 in infant nutrition. The scientific evidence in support of this recommendation is scarce and questionable, as it seems that its clinical impact is little more than a slowing down of gastric emptying.7 However, there is evidence that osmolalities greater than 700mOsm/kg in therapeutic fluids or radiological contrasts are associated with an increased incidence of NEC,9 and therefore a theoretical threshold of 400–600mOsm/kg is widely applied, although there is no evidence to support it.10
Pasteurised and frozen DHM is widely used in neonatal units. It has been demonstrated that these processes affect the osmolality of human milk. While pasteurisation decreases the osmolality, freezing increases it, although not significantly.6 Published data on the kinetics of the changes in osmolality in samples of fortified human milk are contradictory. The most recent studies suggest that the main factor contributing to the increase is the instant addition of solutes that takes place on adding the fortifier,11,12 after which the increase in osmolality over time is not that significant.
In our neonatal unit, the general approach to the nutrition of PT infants is administration of maternal milk or DHM when the mother's own milk is not available, with routine fortification with a multicomponent fortifier and the addition on a case-by-case basis of a modular protein supplement if needed. The protocol of the unit also calls for administration of a multivitamin to patients that do not receive fortified human milk.
We did not find any studies in the literature analysing the increases in osmolality associated with the addition of the fortifiers used in our unit to DHM.
For this reason, we designed this study to analyse changes in the osmolality of DHM after the addition of a multicomponent bovine milk protein fortifier, a modular protein supplement and a multivitamin. In order to match everyday clinical practice, we analysed changes in osmolality through time.
MethodsStudy designProspective study conducted by the departments of neonatology and pharmacy of the Hospital Universitario y Politécnico La Fe in Valencia, Spain, between May 2018 and February 2019. We analysed the osmolality of human milk donated to the regional milk bank (Banco de Leche Materna de la Comunidad Valenciana, BLMCV), which did not meet the criteria established by the bank to be administered to patients, but whose donors signed an informed consent form allowing its use in research.
Analysis of the composition of donor human milkWe analysed the macronutrient contents of the milk (fat, protein and lactose) by infrared transmission spectroscopy with a Miris HMA© analyser (Miris Holding AB, Uppsala, Sweden); the results are presented in Table 1. Samples were thawed, heated to 40°C and homogenised (for 1.5s per 1mL of milk) with an ultrasonic processor (VCX 130; Sonics & Material, Newtown, CT, USA) before the analysis. In adherence with the protocol of the unit, we made a single measurement, and if the value was not in the normal range, we repeated the measurement.
Composition and osmolality of the 6 samples of donor human milk.
| Component | Milk sample | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Protein (g/100mL) | 2.1 | 1 | 0.5 | 1.8 | 0.9 | 0.8 |
| Fat (g/100mL) | 5.2 | 2.6 | 1.6 | 2.4 | 3.5 | 2.7 |
| Lactose (g/100mL) | 6.9 | 7.5 | 7.9 | 6.9 | 7.7 | 7.7 |
| Energy (kcal/100mL) | 85 | 59 | 49 | 59 | 67 | 60 |
| Osmolality (mOsm/kg)a | 310±3.2 | 303±2.6 | 300±1.5 | 295±1.5 | 298±0.6 | 301±1.2 |
Fortification of DHM adhered to the protocol of the unit and manufacturer recommendations. We used the PreNAN FM85© fortifier (Nestlé Infant Nutrition; Vevey, Switzerland), the Resource© Instant Protein supplement (Nestlé Health Science; Vevey, Switzerland) and the Hidropolivit© multivitamin supplement (Grupo Menarini; Badalona, Spain) (compositions presented in Tables 2 and 3).
Composition of the human milk fortifier and modular protein supplement used in the study.
| PreNAN FM 85© (per 100g) | Resource Instant Protein© (per 100g) | |
|---|---|---|
| Protein | 35.5g | 90.3g |
| Carbohydrate | 32.4g | 0.2g (lactose) |
| Fat | 18.1g | 1g |
| Na | 0.9g | 15mg |
| K | 1.2g | 15mg |
| Ca | 1.9g | 1.4g |
| P | 1.1g | 0.74g |
| Mg | 0.1g | |
| Cl | 0.8g | |
| I | 0.4mg | |
| Zn | 23.5mg | |
| Cu | 1.3mg | |
| Fe | 45mg | |
| Se | 93μg | |
| Energy | 435kcal | 371kcal |
| Osmolarity | 339mOsm/La |
Vitamin contents of preparations used in the study.
| Hidropolivit© | PreNAN FM 85© | ||
|---|---|---|---|
| Component | 1mL=28 drops | 4 drops | 1g |
| Retinol (vit. A) | 1500IU | 214IU | 297IU |
| Riboflavin (vit. B2) | 2mg | 0.3mg | 0.05mg |
| Nicotinamide (vit. PP) | 12.5mg | 1.8mg | |
| Pyridoxine (vit. B6) | 1.6mg | 0.2mg | 0.03mg |
| Biotin (vit. H) | 0.125mg | 0.018mg | 0.001mg |
| Ascorbic acid (vit. C) | 50mg | 7.14mg | 5mg |
| Cholecalciferol | 600IU | 85IU | 36IU |
| dl-α-tocopherol acetate (vit. E) | 10mg | 1.4mg | 1mg |
Sources: Menarini and Nestlé Infant Nutrition.
We analysed each of the samples without any additives (fortification A: unfortified DHM) and with 4 different types of fortification: addition of 4 drops of Hidropolivit© multivitamin to 25mL of DHM (fortification B), addition of 1g of PreNAN FM85© to 25mL of DHM (fortification C), addition of 1g of PreNAN FM85© and 0.1g of Resource© Instant Protein to 25mL of DHM (fortification D), and addition of 1g of PreNAN FM85© and 0.2g of Resource© Instant Protein to 25mL of DHM (fortification E).
We stored the DHM frozen at −20°C from the moment of extraction to the moment of its use; DHM was thawed at room temperature before its preparation.
Fortifiers were added with a stepwise procedure, mixing the preparation with a vortex shaker (Vortamix, Argos Technologies, South Scottsdale Court, USA) until the solution became homogeneous.
Osmometer and measurement of osmolalityWe measured osmolality with a Fiske 210 osmometer (Advanced Instruments Inc.; Norwood, MA, USA) that determines the osmolality of solutions using freezing point depression. It uses high-precision thermistors to measure the temperature of the sample, freezing the sample while monitoring for potential overcooling and measuring the freezing point, and it is accurate to 1mOsm/kg H2O.13 Freezing-point depression osmometry is the industry-preferred solution and the criterion standard in clinical chemistry laboratories and pharmaceutical research. It provides quick and accurate measurements and it is the most widely referenced and practiced technique for osmolarity testing.6
We calibrated the instrument with 20μL of Accuref 290 reference solution (Advanced Instruments Inc.; Norwood, MA, USA). We prepared all samples taking aliquots of 25mL, and then transformed the data to calculate values corresponding to 100mL of human milk. To ensure that the aliquots were representative of the DHM, we homogenised the samples (25mL) by mixing for 30s with a vortex shaker (Vortamix, Argos Technologies, South Scottsdale Court, USA). We measured the osmolality of the DHM unfortified and with different types of fortification, making 3 measurements for each sample at baseline (t=0, immediately after addition of the fortifier), 4, 9 and 24h. Between measurements, DHM was stored at 2–8°C, and samples were brought to room temperature before each measurement. Each measurement used 20μL of milk, and we have expressed results as total osmolality in mOsm/kg.
Statistical analysisWe performed the statistical analysis with the software R software (version 3.5.1) and the packages CliCR (version 0.4.22) and lme4 (version 1.1-18-1) (The Freesoftware Foundation, Boston, USA). We have summarised numerical data as mean and standard deviation (SD) and median and interquartile range, and qualitative data as absolute frequencies and percentages. To analyse the changes of the osmolality over time in each aliquot, we fitted a mixed linear regression model, introducing each milk preparation as a random factor and applying the interaction between the type of fortification and time. We took into account the composition of the different samples of DHM and entered the fat, protein and lactose contents as confounding variables. To analyse changes in the milk based on its composition, we fitted a linear regression model in which each milk was a random factor.
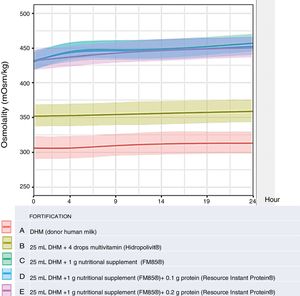
ResultsWe analysed a total of 30 samples of DHM from 6 healthy mothers, unfortified and with 4 fortification schemes. We analysed each sample at 4 time points, taking each measurement 3 times, which resulted in a total of 360 measurements. The mean osmolality of the DHM at baseline (t=0, n=18) was 301±5.2mOsm/kg, which was consistent with the previous literature.4,6,14 The osmolality of DHM (fortification A) stored in a refrigerator at a temperature of 2–8°C increased slightly over time, reaching a mean±SD at 24h of 308.11±5.21mOsm/kg (P=.021), within the safe range for administration to PT infants. The addition of vitamins (fortification B) did not increase the osmolality to a clinically relevant degree compared to unfortified DHM, with a baseline osmolality of 347.33±19.22 that increased over time to up to 354.11±19.12mOsm/kg at 24h, and the differences in the comparison with unfortified milk were not significant (P=.971). The addition of modular protein (PreNAN FM85©) did result in a significant increase in the baseline osmolality that also increased significantly more over time (P<.001, .007 and .007 in the comparison of fortification type A with types C, D and E, respectively), but there were no differences in osmolality between fortification schemes C, D and E (Table 4 and Fig. 1).
Mean±standard deviation of the osmolality (mOsm/kg) at each time point by type of fortification.
| Time point (h)Fortification | 0 | 4 | 9 | 24 | P (comparison of given fortification scheme at different time points) | P (comparison of given fortification scheme to fortification scheme A) |
|---|---|---|---|---|---|---|
| A (n=6)DHM | 301.44±5.20 | 301.56±5.63 | 304.89±4.96 | 308.11±5.21 | .021 | Not applicable |
| B (n=6)DHM+Hidropolivit© | 347.33±19.22 | 348.17±21.44 | 349.67±19.58 | 354.11±19.12 | <.001 | .971 |
| C (n=6)DHM+FM85© | 432.33±14.49 | 446.94±11.56 | 449.94±10.67 | 459.18±11.18 | .01 | <.001 |
| D (n=6)DHM+FM8©+ReIP© (0.1g protein) | 433.94±13.53 | 445.22±8.64 | 447.89±8.24 | 454.28±9.74 | .02 | .007 |
| E (n=6)DHM+FM85©+ReIP© (0.2g protein) | 435±13.98 | 440.28±11.45 | 445.72±11.17 | 453.22±8.15 | .038 | .007 |
DHM, donor human; ReIP, Resource Instant Protein©.
When we fortified DHM with PreNAN FM85© (type C) following the recommendations of the manufacturer, the baseline osmolality at t=0 was 432.33mOsm/kg (SD, 14.49), different from the osmolality reported in the summary of product characteristics (osmolarity=339mOsm/L, which is equivalent to 381mOsm/kg), but it was still within a range considered to be safe.
DiscussionThe high nutritional requirements of PT infants are not fully met by human milk, and therefore fortification is recommended. But fortification has been associated with an increase in gastrointestinal intolerance and gastro-oesophageal reflux.15 The association with more severe disease, such as NEC, is under debate and there is no clear evidence on the subject. Necrotising enterocolitis is a multifactorial disease, and it has been hypothesised that human milk fortifiers could contribute to its development by increasing the osmolality in the intestinal lumen.11 Although there is a growing body of evidence contradicting this hypothesis, caution should be exerted in choosing a fortification scheme to keep from exposing patients to a risk that has yet to be ruled out.7 Early studies on the osmolality of fortified human milk found concerning values higher than the 450mOsm/kg threshold considered safe by the AAP. Today, with the development of new fortification preparations and strategies that sometimes combine several preparations, there is a lack of data on the associated changes in the osmolality of human milk and of banked DHM, which also undergoes processing that affects its osmolality.
A recent survey of several level III neonatal units in Europe and another nationwide survey of neonatal units in Spain revealed substantial variation in human milk processing practices, including fortification.16,17 The differences in organisational structure, staffing, availability of DHM and the products available to fortify milk entail a need to establish protocols for the use of maternal milk and DHM and their fortification, aiming to maximise effectiveness and safety.
The routine approach in our tertiary care unit is to strive to achieve feeding of all very low birth weight PT infants with maternal milk, and DHM sourced from the BLMCV is also available for patients if the mother's own milk is not available or does not suffice to meet the infant's requirements. Before administration, DHM was pasteurised following the protocol of the milk bank (Holder pasteurisation, 62.5°C for 30min) and stored frozen until its administration. We also used a standard fortification strategy, starting out systematically with a multicomponent fortifier, and then adjusting the protein content in select cases with the addition of a modular protein individualised based on the analysis of amino acid metabolism or the nutritional composition of maternal or donor human milk. Individualised fortification is recommended in PT infants that do not meet the expected growth rate, especially those fed with DHM, given the variability in its composition.18 We performed the macronutrient analysis by means of infrared spectroscopy, which had proven useful in a previous study by our group.19 Kreissl et al. reported an increase in the osmolality of human milk of 297–436mOsm/L with the addition of a bovine whey protein fortifier, and an additional increase of 23.5mOsm/L per 0.5g step of protein supplementation.6 This study used protein fortification amounts that far exceeded the recommendations for supplementation in PT infants published by the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN).2 We did not find any data in the literature on the use of the new multicomponent human milk fortifier preparation, PreNAN FM85©, added to banked DHM that has been subjected to pasteurisation and freezing.
Based on the analysis of the samples, we can assert that the maximum level of fortification used routinely in our unit, while exceeding the 450mOsm/kg safety threshold proposed by the AAP with a maximum osmolality of 459.18mOsm/kg,8,18 it never exceeded 600mOsm/kg, which is within the range considered safe for nutrition of PT infants.10
Our findings suggest a tendency towards a decrease in osmolality with the increase of modular protein supplementation. The dosage was +0.1 to +0.2g of protein per 25mL of DHM, which did not result in a significant increase in the total protein content of the sample, and in fact we did not find statistically significant differences between fortification schemes C, D and E. This was consistent with the results of a previous study that reported an average increase in osmolality of 38mOsm/kg with the addition of 1g of hydrolysed protein per 100mL milk,20 the same amount of protein contained in the fortifier used in our study, which would correspond to differences 3.8mOsm/kg between fortification schemes C, D and E in our study, which are not clinically significant and would also account for the absence of statistically significant differences in the analysis.
When it comes to supplementation with a multivitamin, as long as a full dose of enteral nutrition is delivered, with a feed volume greater than 20mL, this is a safe practice that reduces the amount of handling and does not pose a risk of feeding intolerance secondary to an increase in osmolality. Our findings were consistent with those of previous studies.21,22 In case of smaller feed volumes, a possible approach could be to split the total daily dose into smaller doses to reduce the resulting osmolality in each of the feeds. If maternal milk or DHM are fortified with PreNAN FM85© and the infant receives more than 100mL/kg per day, addition of a multivitamin is unnecessary, as recommended vitamin intakes are met by the fortifier and it is better to avoid combining different products.
As for the kinetics of the changes in osmolality, our findings were consistent with those published previously by other authors,11,12 as we found a rapid increase in osmolality right after the addition of the fortifier (86% of the total increase), while refrigeration in the 24h that followed accounted for 14% of the total increase. Fortification accounted for most of the increase in osmolality through the direct addition of solute, as the degradation over time of milk or fortifier components, mainly through the effect of the amylase present in human milk that releases molecules with a higher osmolality (monosaccharides or disaccharides), is a much slower process that thus contributes less to the increase in osmolality and accounts for the increase observed in the preparation over time.11 These data support the recommendation of dedicating an area within neonatal intensive care units to the preparation of PT infant nutrition under safe conditions to prevent contamination. Also, although preparation immediately before administration is preferable, it would be acceptable to prepare the nutrition to be delivered in a 24h-period in a single session per day, by a single member of the staff, allocating to the task the time needed to minimise errors, avoid contamination, etc., which would not be detrimental to the safety of the patient as far as osmolality is concerned.
ConclusionsThe osmolality values found in thawed DHM samples were similar to those reported by other authors. Increases in osmolality resulting from storage of thawed DHM at temperatures of 2–8°C were within the range generally accepted as safe, so it is possible to prepare the milk for PT infants in the neonatal intensive care unit once a day and keep it refrigerated without it resulting in a dangerous increase in osmolality by the time of administration. The increase in osmolality associated with the addition of a multivitamin to DHM free from any other fortifiers does not exceed the threshold considered safe for nutrition of PT infants.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Amparo Ramón and Antonia Gazquez for their contribution in obtaining the samples of DHM and their nutritional analysis, and Antonio J. Cañada Martínez for his help in the statistical analysis.
Please cite this article as: Torres Martínez E, García Robles AA, Gormaz Moreno M, Gimeno Navarro A, Izquierdo Macián I, Poveda Andrés JL, et al. Efecto de la adición de fortificantes y de módulo de proteínas en la osmolalidad de la leche materna donada. An Pediatr (Barc). 2020;93:297–304.
Previous presentation: brief oral presentation at the XXVII Congress of Neonatology and Perinatal Medicine; October 2–4, 2019; Madrid, Spain.