Hereditary fructose intolerance (HFI, MIM #229600) is an autosomal recessive disease due to Aldolase B deficiency, enzyme responsible for fructose metabolism mainly in the liver. The consumption of fructose, sucrose, sorbitol or tagatose1 for HFI patients causes severe symptoms which can lead to analytical, neurological, hepatic and renal alterations, hypoglycemia and even death.2
Oral liquid presentations of drugs are classically made with sucrose (simple syrup) but the use of other sweeteners is being increased, such as hydrogenated syrups (polyalcohols) or, in a lesser extent, glucose syrups. The polyalcohols (maltitol, sorbitol, lactitol, etc.) are obtained by sugars’ catalytic hydrogenation leading to products with low caloric power. Otherwise, glucose syrups are more caloric, due to their lower sweeting power, although it can be increased with the transformation of a part of the glucose in fructose (isomerization). The food industry should indicate the amount of fructose in glucose in the definition of the ingredients if it is greater than 5%.3 In the pharmaceutical industry, it is mandatory to put a warning for HFI patients in leaflet and the label if the product contains sweeteners contraindicated in HFI such as fructose, sucrose, inverted sugar, sorbitol, maltitol, isomalt and lactitol4 (Table 1). We have detected errors in the denomination of glucose syrups in drug labels potentially harmful for HFI patients.
Characteristics of excipients/sweeteners and their tolerance in HFI.5,6
| Excipient | Composition | Synonyms | Obtaining | Suitable in HFI? |
|---|---|---|---|---|
| Monosaccharides | ||||
| Sorbitol | Sorbitol | d-glucitol, d-sorbitol | Glucose hydrogenation | No |
| Disaccharides | ||||
| Sucrose | Glucose-fructose | Saccharose, sugar, common sugar | From the sugar cane | No |
| Isomalt | Mix of maltitol and glucose-manitol | Hydrogenated isomaltulose, hydrogenated palatinose | Isomaltulose hydrogenation | Noa |
| Lactitol | Galactose-sorbitol | Lactit, lactositol, lactoboost | Lactose hydrogenation | Noa |
| Maltitol | Glucose-sorbitol | Hydrogenated maltose | Starch hydrolysate hydrogenation | Noa |
| Syrups | ||||
| Invert sugar | Mix of glucose and fructose | Invert liquid sugar, invert syrup sugar | Sucrose hydrolysis | No |
| Glucose syrup | Mix of glucose, maltose and oligosaccharides | Corn sugar | Starch hydrolysis | Yesb |
| Maltitol syrup | Mix of sorbitol, maltitol and hydrogenated polysaccharides | Hydrogenated maltose syrup, hydrogenated glucose syrup with high maltose, hydrogenated starch hydrolysate | Glucose syrup or starch hydrolysate hydrogenation | Noa |
| High fructose corn syrup | Glucose and fructose | Isoglucose | Corn starch hydrolysis and isomeration of some glucoses in fructoses (42–55%) | No |
To establish the scope, we have revised the drug label of medicines with glucose syrup between May 2013 and July 2016 using the “Prescription Nomenclator” tool of the Spanish Medicines Agency (www.aemps.gob.es) and the information has been confirmed with each manufacturer laboratory.
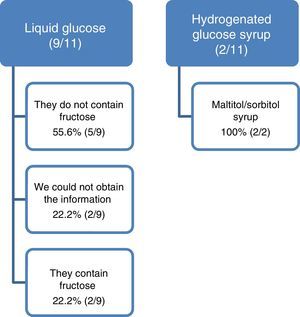
We detected 42 presentations commercialized with glucose syrup. We excluded 4 topical and 27 presentations which were also containing sucrose, sorbitol, maltitol, isomalt or high fructose corn syrup (3 presentations did not have the alert for HFI (11%)). We analyzed 11 presentations: 9 with liquid glucose and 2 with hydrogenated glucose syrup.
In 2 presentations with liquid glucose and in 2 with hydrogenated glucose syrup, the laboratory confirms that they contain fructose and maltitol/sorbitol respectively. The formulation of these 4 presentations is syrup or oral solution; the others are tablets, capsules or vials. In one of them the laboratory indicates that the glucose syrup contains 40% of fructose. In 2 presentations from a same laboratory, we could not obtain the information of the liquid glucose composition and in another 3 (27.7%) the first response was imprecise or contained errors that required a second consultation (Fig. 1). In some cases the delay in the reply has been four months.
In conclusions, we detected very serious errors in the information about excipients in drugs labels that carry a serious safety problem for patients with HFI. The two most serious mistakes have been:
- -
Indicate in drug label “glucose syrup or liquid glucose” when in fact it is “glucose-fructose syrup”.
- -
Indicate in drug label “hydrogenated glucose syrup” as synonymous with “glucose syrup”, when in fact it is “maltitol syrup or sorbitol syrup”.
In addition, it has been observed that not all medications with fructose, sucrose, sorbitol, maltitol, isomalt or lactitol contain the warning or contraindication mandatory for HFI patients in packaging material, label and leaflet (according to legislation).
The review has been done through an application that filters the drugs according to excipients. However, it cannot be guaranteed that all medications with glucose syrup have been checked. Therefore, these errors have been notified to the Patient Safety Program and Medication Errors of the Madrid Regional Health Sistem and to the Spanish Medicines Agency (errors.etiquetado@aemps.es), so that they make the necessary notifications to the pharmaceutical industry and to the health professionals.
In spite of the size of the sample, both the errors in drug label and the difficulty in obtaining reliable and accurate information for the manufacturer are very striking, being necessary, in several cases, to request further clarifications, which have led to corrections with respect to the information previously issued. In some cases, if the first manufacturer's information had been considered correct, the consequence of the administration of the drug in an HFI patient could have had serious consequences on his health. We believe there is a necessity of a review process on the contents of the label in a coordinated way between regulatory agencies and the companies that manufacture and/or market these medicines.
Please cite this article as: Izquierdo-García E, Escobar Rodríguez I, Moreno-Villares JM, Iglesias Peinado I. Jarabes de medicamentos: errores en ficha técnica con posibles consecuencias en pacientes con intolerancia hereditaria a la fructosa. An Pediatr (Barc). 2017;87:351–353.