Surgical intervention in necrotising enterocolitis (NEC) is correct when there is intestinal gangrene. This is evident when gangrene produces perforation and pneumoperitoneum, with this being the only universally accepted radiological indication for the surgical intervention of NEC.
ObjectiveTo perform an analysis on patients with surgically managed NEC, including determining how the decision to intervene is reached, the outcomes, and if patients with perforation had a pneumoperitoneum.
MethodsRetrospective review of neonates with surgical NEC over a period of 10years (2006–2015). An analysis was made of pre-surgical X-ray findings, which were compared with surgical ones, in addition to the morbidity and mortality, depending on the presence (N+) or absence (N−) of pneumoperitoneum. An evaluation was also made of the interobserver concordance with a paediatric radiologist blinded to the clinical reason using the kappa agreement index.
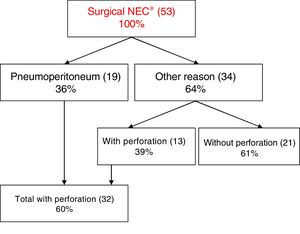
ResultsA total of 53 neonates were included in the study. Surgical treatment was indicated after observing pneumoperitoneum in 36%. In the remaining neonates, the surgical decision was made after noting a clinical and metabolic deterioration with classical X-ray findings. Intestinal perforation was observed in 39% of the N− neonates.
There were no statistical differences between either group on analysing the excised intestinal length, days of intubation, starting of enteral nutrition, and the mortality rate. Comparisons in terms of duration of symptoms and total hospital stay were statistically significant (7 vs. 2 days, p=0.008; 127 vs. 79 days, p=0.003, respectively), with both being more favourable in the N+ group. These differences remained when the groups were adjusted by birthweight.
ConclusionsSurgical indication has to be done on an ensemble of clinical and radiological evidence, as 39% of the neonates in the N− groups were perforated.
In our study, the presence of a pneumoperitoneum did not correlate with a worse prognosis.
La intervención quirúrgica en las enterocolitis necrosantes (EN) es precisa cuando existe gangrena intestinal, hecho evidente cuando produce perforación y neumoperitoneo, siendo este la única indicación radiológica aceptada universalmente para la intervención quirúrgica.
ObjetivoAnalizar a los pacientes intervenidos de EN, saber por qué se les intervino, cómo evolucionan y si los pacientes perforados presentan neumoperitoneo.
MétodoEstudio retrospectivo de una cohorte de recién nacidos con EN intervenidos durante un periodo de 10 años (2006-2015). Se analizan los hallazgos radiológicos preoperatorios y se correlacionan con los quirúrgicos y con la morbimortalidad, dependiendo de la presencia de neumoperitoneo (N+) o no (N–). Se evaluó la concordancia interobservador con radiólogo pediátrico enmascarado a la clínica mediante el índice de acuerdo kappa.
ResultadosSe analizó a 53 pacientes. El 36% se intervino tras la visualización de neumoperitoneo; en el resto, la indicación fue deterioro clínico y metabólico, junto con hallazgos radiológicos asociados. En el 39% del grupo N– se objetivó perforación.
No se encontraron diferencias significativas en ambos grupos con respecto a longitud intestinal resecada, días de intubación, día de inicio de nutrición enteral y mortalidad. La comparación entre duración de síntomas y estancia hospitalaria total en ambos grupos (N–/N+) fue significativa (7 vs. 2 días, p = 0,008; 127 vs. 79 días, p=0,003 respectivamente), siendo más favorable en el grupo N+. Estas diferencias se mantuvieron al ajustar por peso.
ConclusionesLa indicación quirúrgica ha de basarse en un conjunto de datos clínicos y radiológicos, ya que el 39% de los pacientes sin neumoperitoneo presentaron perforación.
En nuestro estudio la presencia de neumoperitoneo no se correlaciona con peor pronóstico.
Necrotising enterocolitis (NEC), first described by Siebold in 1825,1 is the most common gastrointestinal emergency occurring in neonatal intensive care units (NICUs),2 with an estimated incidence of 0.3–3 cases per 1,000 live births.3,4 Its aetiology is not well understood, although it is hypothesised that it is related to decreased perfusion and ischaemia of the intestinal wall (particularly in immature intestines), leading to disruption of the intestinal barrier and enabling bacterial passage and activation of inflammatory mediators.5 The main risk factors for the development of this disease are preterm birth, perinatal asphyxia, early enteral feeding in preterm newborns, congenital heart defects and umbilical catheterization, among others.5
Necrotising enterocolitis should not be confused with focal intestinal perforation, which is less frequent and affects up to 2% of extremely low birth weight infants.3 Other differential features of focal intestinal perforation are: lack of systemic involvement, absence of intestinal pneumatosis, earlier onset, lower birth weight and extreme prematurity.3
Necrotising enterocolitis is classified according to the staging criteria proposed by Bell et al. as stage I (suspected), ii (proven) or iii (advanced),6 a system that was later modified by Walsh and Kliegman in 1986.7 In this modified scheme, stages are further subdivided into A or B depending on the radiographic findings: stage IA or B (normal or intestinal dilation), stage IIA (ileus, pneumatosis intestinalis) or IIB (portal venous gas), stage IIIA (ascites) or IIIB (pneumoperitoneum).5,6 Another classification scheme was published in the Vermont Oxford Network Manual of Operations that described the clinical and radiographic findings required to establish the diagnosis of NEC.8
There is widespread agreement that surgical intervention is necessary in patients with NEC that have developed intestinal gangrene, whose presence is evident if it leads to perforation and pneumoperitoneum, which is not always the case.9 Free air in the abdominal cavity is the only radiographic feature universally accepted as an indication for surgical intervention in NEC.10
The aim of this study was to analyse the cases of NEC treated with surgery to establish the reasons that led to performance of surgery, to determine whether pneumoperitoneum had been present in all patients with bowel perforation, and to assess patient outcomes based on whether pneumoperitoneum had or had not been present before surgery.
To this end, we compared the radiographic findings (whether pneumoperitoneum was present or absent) with the surgical findings and the outcomes in a group of patients with NEC that underwent surgical intervention.
Sample and methodsWe conducted a retrospective study in a cohort of newborns with NEC that underwent surgery between January 2006 and December 2015. We divided these patients into 2 groups for comparison based on the preoperative presence or absence of pneumoperitoneum (P+/P–). We compared both groups based on the surgical findings, which included the length of resected bowel and the presence or absence of NEC totalis (involvement of 3 or more bowel segments). We also compared other variables related to morbidity and mortality: duration of symptoms from onset to the day of surgery, days of intubation, days of total parenteral nutrition, days from surgery at initiation of enteral feeding, length of stay in days, and mortality.
In addition, we assessed interrater reliability, using the kappa statistic to compare the interpretation of the preoperatory findings made a posteriori by an expert paediatric radiologist masked to the symptoms to the preoperatory assessment made jointly by the neonatologist and the on-call paediatric surgeon. A kappa value of 0 signifies a total absence of interrater agreement, while a value of 1 signifies perfect agreement.4 We assessed the level of agreement applying the criteria proposed by Landis and Koch: k<0.20 (very poor), 0.21<k<0.40 (poor), 0.41<k<0.60 (fair), 0.61<k<0.80 (good), and 0.81<k<1.00 (excellent).11
To compare qualitative variables, we used the chi square test or the Fisher exact test as appropriate. We compared quantitative variables by means of the nonparametric Mann–Whitney U test. We assessed survival using the Kaplan–Meier method.
We set the alpha level to define statistical significance at 0.05. The statistical analysis was performed with the software SPSS version 23.0.
ResultsIn the 10 years under study, 53 patients with NEC underwent surgical intervention. They had been born at a median of 28+6 weeks’ gestation (range, 23–41 weeks) with a mean birth weight of 1229±581g (range, 590–3087g), with 94.3% being born with a low birth weight (50 newborns with weight <2500g), 73.6% with a very low birth weight (39 newborns with weight <1500g) and 50.9% with an extremely low birth weight (27 newborns with weight <1000g). We estimated an incidence of surgical NEC of 7 newborns per 10,000 live births.
In this cohort, 51% of patients were female (n=27) and 94.3% had been born preterm (born before 37 weeks’ gestation, n=50), with 77.4% born very or extremely preterm (born before 32 weeks’ gestation, n=41).
The mean age at the time of surgery was 19±14 days (range, 2–63 days), and the mean weight was 1474±669g (range, 650–3750g).
The sensitivity of abdominal radiography for the diagnosis of pneumoperitoneum is 54.5%, while the specificity is 92.3%. In 36% of patients (n=19), surgery was performed due to the presence of pneumoperitoneum, while in the remaining patients (n=34) it was performed for other reasons (clinical deterioration, metabolic derangement or intestinal obstruction) (Fig 1).
When we analysed the two groups defined by the presence or absence of pneumoperitoneum (P+/P–), we found a shorter duration of symptoms from onset to surgery in the P+ group, with a mean of 2±1.7 days (range, 1–3 days) compared to 7±8 days in the P– group (range, 3–10 days), and also a shorter length of stay, which was of 79±28 days in the P+ group (60–98) compared to 127±43 days in the P– group (103–150) (excluding deceased patients), differences that were statistically significant (p=0.008 and p=0.003, respectively) (Table 1). The differences remained significant when we adjusted the analysis for weight: in the group with birth weights of more than 1000g (+1000g), the mean length of stay was 58±10 days in P+ newborns (range, 48–69) compared to 89±15 days in the P– group (range, 71–107) (p=0.005) (Table 1). We also found differences in the group with birth weight of less than 1000g, although they were not statistically significant: mean of 107±20 days (range, 78–135) in the P+ group vs. 146±43 days (range, 116–175) in the P– group (p=0.064).
Statistical analysis of quantitative data in the two groups compared in the study.
| Variables | P+ | P– | p |
|---|---|---|---|
| Duration of symptoms in days (mean±SD) | 2±1.7 | 7±8 | 0.008 |
| Days of intubation (mean±SD) | 7±11 | 4±2 | 0.59 |
| Days post-surgery at initiation of EF (mean±SD) | 6±3 | 11±13 | 0.24 |
| Length of stay in days (mean±SD) | 79±28 | 127±43 | 0.003 |
| Length of stay in ≥1000g | 58±10 | 89±15 | 0.005 |
| Length of stay in ¿1000g | 107±20 | 146±43 | 0.064 |
| Resected length of bowel in cm (mean±SD) | 13±9 | 20±11 | 0.21 |
| Mortality (n – %) | 10 (45%) | 12 (55%) | 0.85 |
| Mortality in ≥1000g | 7 (54%) | 6 (46%) | 0.6 |
| Mortality in ¿1000g | 4 (40%) | 6 (60%) | 0.53 |
Data expressed as means and p values.
EF, enteral feeding.
Statistically significant results are presented in boldface.
When we analysed mortality, we did not find statistically significant differences between the two groups. The overall mortality was 41.5%, corresponding to 10 newborns in the P+ group (45%) and 12 of the P– group (55%). We also found no statistically significant differences in any of the other variables under study (Table 1).
The kappa statistic was 0.7, which corresponds to a good correlation based on the criteria proposed by Landis and Koch.
DiscussionNecrotising enterocolitis is one of the main causes of morbidity and mortality in newborns.12
Most cases of NEC occur in preterm newborns. The mean gestational age at birth in our cohort was 28+6 weeks (range, 23–41), which was consistent with previous studies.13,14
The mean birth weight in our cohort was 1229±581g, with a range of 590–3087g, which was similar to the findings reported in the literature,15 although there seems to be a declining trend in the mean birth weight of patients with surgical NEC, as suggested by other authors.9
The mean age at the time of surgery in our study was 19 days (range, 2–63), which was similar to the one reported in a study conducted in 200216 and another study we found in the reviewed literature.13
When medical treatment fails (including antibiotherapy, discontinuation of enteral feeding and supportive care),17 the decision to perform surgery is based on radiographic findings and clinical manifestations suggestive of metabolic derangement.2,8
In patients with suspected NEC, a radiological evaluation can confirm the staging as proven or advanced.18 Extraluminal air (free air) is a sign of advanced NEC8 and the sole radiologic finding that is universally accepted as an indication for surgical intervention,19 as it is direct evidence of perforation. Some authors consider metabolic derangement a sufficient indication for surgery and have established a series of laboratory criteria and thresholds to determine the appropriateness of intervention, even in the absence of pneumoperitoneum.3 We ought to highlight the importance of obtaining the two radiographic images (antero-posterior view and horizontal beam view with the patient in the supine position) within 48h from onset, as most perforations develop in that time interval,10,12 something that we corroborated in our results, as we found that a mean of 2 days elapsed from the onset of symptoms to evidence of pneumoperitoneum, with a range of 1–3 days.
Pneumoperitoneum is not always present and is found in 50–75% of patients with intestinal perforation due to NEC,20 with other authors reporting its presence in as few as 12–50% of these patients.18,21 Thus, the sensitivity of this radiographic finding is much lower than expected. In our study, its sensitivity was 54.5%. There are several possible explanations for the absence of pneumoperitoneum in cases of intestinal perforation: (1) perforation of the jejunum or ileus, which may not contain air; (2) early closure of perforation; (3) retroperitoneal perforation; (4) small amount of gas due to effective suction through a nasogastric tube; (5) lack of an optimal radiographic view showing the pneumoperitoneum, and (6) perforation occurring after obtaining the radiograph and before surgery.1
This radiographic feature is not always easy to identify, and there are several radiographic signs that help detect its presence: Rigler's sign (gas on both sides of the intestinal wall), the falciform ligament sign (gas outlining the falciform ligament), the football sign (gas outlining the peritoneal cavity), the inverted-V sign (gas outlining the medial umbilical folds) and right-upper-quadrant gas sign (localised gas in this quadrant, in the subhepatic space).22
Some critically ill infants require mechanical ventilation, which may cause pneumothorax or pneumomediastinum and therefore pneumoperitoneum. In these patients, it is important to perform a correct differential diagnosis of gastrointestinal perforation and medical pneumoperitoneum to avoid unnecessary surgical procedures.23 We found one such patient in this study. As we mentioned before, pneumoperitoneum was the indication for surgery in 39% of cases, a proportion that was similar to those reported in other studies.24,25
In the past, there was widespread agreement that the prognosis of patients with NEC worsened once intestinal perforation occurred,1,10,26 although the published evidence is not completely concordant on this aspect.24,27 As Grosfeld et al. already stated in 1991, early diagnosis, advances in neonatal intensive care and prompt surgical intervention when indicated have improved survival in patients with NEC.28
In our study, we found that even adjusting for weight, radiographic evidence of pneumoperitoneum was not in and of itself associated to poorer outcomes, but rather the opposite. This was probably due to the association of pneumoperitoneum with a shorter duration of symptoms before surgery compared to patients without pneumoperitoneum. In many cases, the decision to perform surgery in preterm infants with manifestations suggestive of NEC is delayed for days while the patient receives more aggressive medical treatment, precisely because there are no radiographic findings that clearly “invite” the surgeon to intervene.
Considering the low sensitivity of abdominal radiography found in our study, relying on the detection of free abdominal air as the sole definitive indication for surgery should by now be considered obsolete or risky. Other authors also recommend against waiting for stage IIIB NEC to operate, given that it may not always be detected radiographically, as long as the patient develops metabolic derangement or infection despite adequate medical treatment.
In our study, 10 patients in the pneumoperitoneum group (45%) and 12 in the group without pneumoperitoneum (55%) died. The overall mortality was 41.5%, a proportion that was similar to those reported in other case series, which range between 55% and 83%.5,13
The kappa statistic found in this study revealed a very good interrater reliability (0.7). Nevertheless, to decrease both false-negative and false-positive interpretation, it is recommended that an experienced radiologist always review the radiographs of patients with suspected NEC.29
The authors of a study conducted in 2002 estimated the sensitivity and specificity of pneumoperitoneum at 52% and 92%, respectively,30 which are practically the same as the estimates obtained in our study.
The limitations of this study include its retrospective design and the small sample size, and a prospective study would be necessary to collect more reliable information regarding patient outcomes that would allow drawing firmer conclusions.
ConclusionGiven that there is no specific clinical, radiologic or laboratory finding to establish the need for surgery and that (as occurred in 39% of the patients in our study) perforation may be present in the absence of pneumoperitoneum, the decision to operate must be based on the collection of clinical and radiologic features of the patient.
We have not found other articles comparing the outcomes of patients that underwent surgery for NEC based on the presence or absence of pneumoperitoneum, so we believe that our study contributes relevant information to our understanding of the outcomes of NEC.
The presence of pneumoperitoneum in cases of NEC is not associated with a less favourable outcome.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villamil V, Fernández-Ibieta M, Gilabert Ubeda MA, Aranda García MJ, Ruiz Pruneda R, Sánchez Morote JM, et al. Correlación entre el neumoperitoneo y los hallazgos quirúrgicos y morbimortalidad en recién nacidos con enterocolitis necrosante. An Pediatr (Barc). 2018;89:205–210.