The aims of the study are to evaluate the efficacy and safety of continuous subcutaneous insulin infusion (CSII) treatment in pre-school children with type I diabetes, and to assess whether the criteria of good metabolic control are achieved.
MethodA review was performed on the medical charts of patient's <6 years of age who started CSII treatment between 2003 and 2014. The cohort consisted of 27 patients (mean age 4 (2.9–4.7) years, 56% males). An analysis was made including the age at onset, type I diabetes duration, HbA1c (HPLC, Menarini, normal value 5.1±0.31%), insulin dose (IU/kg/day), number of capillary blood glucose measurements, number of baseline processes per day, % baseline/total insulin (B/TI), insulin ratios (I/HC) at different meals, severe hypoglycaemia (HS episodes/100 patients years), DKA events, percentages of normal blood glucose (70–180mg/dL), hyperglycaemia (>180mg/dL), and hypoglycaemia (<70mg/dL), mean blood glucose, standard deviation and coefficient of variation ((SD/mean glucose)×100). Statistical analysis was performed using SPSS.
ResultsHbA1c decreased from 6.9% (6.7–7.5) to 6.8% (6.4–7.1) after one year of CSII. Afterwards, it remained under 6.8% during the follow-up (median 5 years [3–6]). Prior to CSII, 74% of children had HbA1c levels <7.5%. It increased to 96% after one year of CSII. Median blood glucose measurements/day was 10 (9–11). Total insulin dose did not change significantly. During the follow-up, there was one episode of DKA and one episode of HS. I/HC at breakfast were higher than at other meals (0.92 vs. 0.55, 0.6 and 0.5, respectively).
ConclusionsCSII is effective and safe in pre-school children. It allows good metabolic control (based on Society for Paediatric and Adolescent Diabetes/American Diabetes Association criteria) to be achieved and maintained for long periods of time without an increase in adverse events.
Valorar la eficacia y la seguridad del tratamiento con infusión subcutánea continua de insulina (ISCI) en niños menores de 6 años durante periodos prolongados de tiempo y evaluar si alcanzan criterios de adecuado control glucémico.
MétodosEstudio retrospectivo de 27 niños que iniciaron tratamiento con ISCI entre 2003–2014. Edad de inicio de ISCI: 4 años (2,9-4,7); 56% varones. Se recogen: edad de inicio de diabetes, tiempo de evolución de diabetes, HbA1c (HPLC Menarini, valor normal 5,1±0,31%), dosis insulina (u/kg/día), número de controles de glucosa capilar/día, número de tramos basales/día, porcentaje de insulina basal, ratios insulina/ración hidratos carbono (I/HC), episodios de hipoglucemia grave y de CAD (episodios/100 pacientes-año), porcentajes de normoglucemia (70-180mg/dl), hiperglucemia (>180mg/dl) e hipoglucemia (<70mg/dl), glucemia media, desviación estándar y coeficiente variación ([desviación estándar/glucemia media]×100). Análisis estadístico por SPSS.
ResultadosLa HbA1c disminuye de 6,9%(6,7-7,5) a 6,8%(6,4-7,1) el primer año, posteriormente se mantiene <6,8% durante el periodo de seguimiento (mediana 5 años [3-6]). Antes de ISCI el 74% tenían HbA1c <7,5% y al año el 96%. Media de controles de glucosa capilar/día de 10(9-11). No se observaron cambios significativos en la dosis de insulina. Hubo un episodio de CAD y un episodio de hipoglucemia grave durante el seguimiento. La ratio I/HC al desayuno fue superior a la de otras ingestas (0,92u/r vs. 0,55, 0,6 y 0,5 en comida, merienda y cena).
ConclusionesEl tratamiento con ISCI es eficaz y seguro en menores de 6 años durante periodos prolongados de tiempo. Permite alcanzar los objetivos de buen control metabólico recomendados por la Asociación Americana de Diabetes y la Sociedad Internacional de Diabetes Pediátrica y del Adolescente, sin incremento de efectos adversos.
The Diabetes Control and Complication Trial1 showed that achieving good metabolic control is necessary to prevent or delay the development of chronic complications in type 1 diabetes (T1D). Different studies have demonstrated that these complications already start to develop in childhood,2–4 so that adequate metabolic control should be maintained from the time of diagnosis in this age group, too. Furthermore, there is increasingly conclusive evidence of the vulnerability of the developing brain to hypoglycaemia, chronic hyperglycaemia and fluctuations in blood glucose, which have been associated with smaller white and grey matter volumes.5–7 For all the above reasons, the current guidelines of international diabetes societies state that the primary objective of diabetes treatment in the paediatric age group should be the achievement of blood glucose levels as close to normal as possible while maintaining haemoglobin A1c (HbA1c) under 7.5% throughout childhood.8,9 However, this goal seems difficult to achieve and maintain in everyday clinical practice.10–12
The treatment of diabetes in the youngest patients poses specific challenges associated with the characteristics of this age group, such as a high sensitivity to insulin, irregular eating and physical activity patterns, the inability to report manifestations of hypoglycaemia, and a stronger impact of glucose fluctuations on neurocognitive functioning.13
At present, continuous subcutaneous insulin infusion (CSII) is the method of insulin administration that best simulates the physiologic pattern of pancreatic secretion, with a basal rate that is adjusted in time intervals throughout the day and delivery of different boluses for mealtime doses or correction of high blood sugar. In young children, this method allows the delivery of very small and variable basal rates to minimise the risk of nocturnal hypoglycaemia, the splitting of mealtime boluses in children who are poor eaters (administering part of the bolus before the meal and then completing the dose needed based on actual intake), or the administration of small boluses to correct hyperglycaemia.
Most studies in children treated with CSII have shown improvements in HbA1c, primarily at the time of treatment initiation.14–16 The duration of followup in most of these studies has been short. Although several recently published studies collected data over longer periods of time and found improvements in HbA1c,17 the reported levels exceeded the targets recommended by scientific societies.12,18
The objective of our study was to assess whether treatment with CSII is efficacious and safe in children aged less than 6 years in the long term, and whether it can maintain good metabolic control based on the criteria established by the International Society for Pediatric and Adolescent Diabetes (ISPAD), the American Diabetes Association (ADA) and the International Diabetes Federation (IDF).8,9
Materials and methodsWe reviewed the health records of all children with T1D that started treatment with CSII between 2003 and 2014 and who were aged less than 6 years at the time of initiation of CSII.
Data for the following variables were collected in each medical visit: age, sex, height, weight, HbA1c, total daily dose of insulin (IU/kg/day), basal insulin percentage of the total daily dose, insulin to carbohydrate ratio (I/HC), number of basal rates per day, number of capillary blood glucose (CBG) measurements per day, episodes of severe hypoglycaemia defined as severely low blood glucose with altered mental status, seizures or coma and expressed as the number of episodes per 100 patients per year, and episodes of diabetic ketoacidosis (DKA). The level of HbA1c was measured with a HPLC analyser (Menarini; normal range, 5.1±0.31%) calibrated according to the Diabetes Control and Complication Trial and IFCC protocols. For the purpose of analysis, we used the mean of the last 3 HbA1c values. The data stored in glucose meters and insulin pumps were downloaded and analysed at each medical visit, collecting information on the mean blood glucose level and its standard deviation, and percentages of normal blood glucose (70–180mg/dL), hypoglycaemia (<70mg/dL) and hyperglycaemia (>180mg/dL). We calculated the coefficient of variation (CV) of blood glucose with the following formula: (standard deviation/mean)×100.
The main goals in switching to CSII treatment were to achieve better metabolic control (reduction of HbA1c and/or changes in glucose levels), reduce the number of hypoglycaemic episodes and improve quality of life.
Treatment with CSII was initiated following the protocol of our diabetes unit.19 Prior to initiation of CSII, all patients, their families, and individuals in charge of their care completed a diabetes education programme on CSII. The programme started with the evaluation of their general knowledge on the management of diabetes a week before initiating the new therapy. The CSII education programme consists of 25h of training over 4 consecutive days. Use of the infusion pump set started on day 2. All patients performed carbohydrate counting prior to initiation. During the 4 days of training, blood glucose levels were measured frequently to adjust the basal insulin rates and to calculate the I/HC ratios for each meal. The insulin sensitivity index was also calculated for each time segment. Correction boluses were administered whenever the general goals for adequate glycaemic control recommended by international diabetes societies were not met.8 The infusion set insertion site was in the buttocks.
Children and their families were given the option to communicate with the diabetes team by telephone on a daily basis. Follow-up visits were scheduled on weeks 1 and 4 from initiation of CSII. From that point, routine checkups at the diabetes unit took place every 2–3 months. The data stored in the insulin pump and glucose meter were downloaded during each medical visit and analysed in depth with the patient and the family. The medical team assessed whether glycaemic control goals were achieved, and whether further training by health educators was necessary. The infusion set was changed every 2–3 days.
The study was approved by the Ethics Committee of the Hospital Ramón y Cajal. We obtained the informed consent of patients and their parents.
Statistical analysisWe performed the statistical analysis with the software SPSS (Evanston, IL) version 21 for Windows. We have summarised quantitative variables as median, interquartile range (Q1–Q3) and range (minimum–maximum). We have described qualitative variables as absolute and relative frequencies.
We used nonparametric tests due to the size of the study sample. The patients acted as their own controls, which eliminated potential sources of confounding. We compared the follow-up data with baseline data collected before initiation of CSII. We defined statistical significance as a p-value of less than 0.05.
ResultsDemographic dataThe final number of children aged less than 6 years treated with CSII for more than 1 year was 27 (56% male). The mean age at initiation of CSII was 4 years (2.93–4.67). Six children started the new treatment before age 3 years. The mean duration of diabetes prior to initiation of insulin pump treatment was 1.58 years (0.92–2.10). Two children started CSII therapy before 6 months had elapsed from the onset of diabetes (at ages 11 months and 3 years, respectively); to minimise the potential effects of the honeymoon phase in these children, we used the measurement of HbA1c performed right at the time of treatment initiation as the baseline. All children were previously on a basal-bolus insulin regime (multiple daily injections [MDI]). The basal insulin was insulin detemir in 23 children, NPH insulin in 3, and insulin glargine as a single dose in 1.
Prior to initiation of CSII, 74% of patients met the ISPAD/IDF/ADA criteria for adequate metabolic control, with HbA1c levels of less than 7.5%.
Table 1 presents the demographic characteristics of patients at initiation of CSII.
Demographic data from the visit prior to initiation of CSII and the last recorded visit. The HbA1c value is the mean of the last 3 measurements.
| Visit prior to initiation of CSII | Last follow-up | |
|---|---|---|
| Age (years) | 4 (2.23–4.67) | 8.5 (5.9–9.67) |
| Duration of DM (years) | 1.58 (0.92–2.1) | 5.8 (3.8–7.6) |
| Duration of CSII followup (years) | – | 5 (3–6) |
| HbA1c (%) | 6.9% (6.7–7.5%) | 6.8% (6.4–7.1%)* |
| Insulin dose (IU/kg/day) | 0.61 (0.45–0.78) | 0.67 (0.41–0.97) |
| Basal insulin (%) | 40% (36–50%) | 36% (26–47%) |
| Met ISPAD/ADA criteria for good control (%) | 74% | 93.5% |
The baseline HbA1c level was 6.9% (6.7–7.5%) (52 [50–57]mmol/mL IFCC). After 1 year of treatment, the HbA1c level changed to 6.8% (6.4–7.1%) (51 [47–54]mmol/mL IFCC) (P 0.004). In the first year, HbA1c improved in 70% of patients and was maintained in 7%. By the end of the first year, only one child had HbA1c values of 7.5% or higher. This initial improvement was sustained through every year of followup (Table 2), with maintenance of a mean HbA1c of 6.8% or less throughout the followup. A cross section of the sample in the last year of treatment with CSII found a median HbA1c of 6.8% (6.4–7.1%) (51 [47–54]mmol/mL IFCC), with a statistically significant difference compared to HbA1c at initiation of CSII (Table 1). Ninety-five percent of children achieved the metabolic control goals recommended by international diabetes societies for the paediatric population through the entire followup (median duration of 5 years [3–6]; range, 1–9).
Changes in HbA1c, mean blood glucose, SD of mean blood glucose, coefficient of variation (CV%); percentage of hypoglycaemia (<70mg/dL), normal blood glucose (70–180mg/dL) and hyperglycaemia (>180mg/dL), episodes of DKA and severe hypoglycaemia during treatment with CSII.
| Years of followup | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| N | 27 | 27 | 24 | 20 | 17 | 14 | 7 |
| HbA1c (%) | 6.9% (6.7–7.5%) | 6.8%** (6.4–7.1%) | 6.6%*** (6.3–7%) | 6.7%*** (6.2–6.9%) | 6.6%** (6.2–7.1%) | 6.6%* (6.2–7.1%) | 6.6%* (6–7.3%) |
| Blood glucose mean ± SD (mg/dL) | 150±73 | 151±71.5 | 148.5±71 | 145±67 | 147±65 | 148.5±60 | 136±60 |
| Coefficient of variation (%) | 47% | 48.5% | 46.6% | 47.3% | 45.8% | 47% | 44.1% |
| Mild hypoglycaemia (%) | 9% | 11% | 9% | 11% | 9.5% | 9% | 11% |
| Normal glucose (%) | 48% | 54% | 55% | 52% | 56% | 58% | 56% |
| Hyperglycaemia (%) | 40% | 33% | 35% | 39% | 34% | 29% | 36% |
| Severe hypoglycaemia (episodes/year) | 3 | 1 | 0 | 0 | 0 | 0 | 0 |
| DKA (episodes/year) | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Met ISPAD/ADA criteria for good control (%) | 74% | 93.5% | 92% | 100% | 88% | 100% | 100% |
The median number of CBG measurements per day at 1 year of CSII was 10 (9–11); data was not available for every patient on the number of CBG measurements per day before initiation of CSII.
When it came to the data on the mean blood glucose and its standard deviation and the percentages of mild hypoglycaemia, hyperglycaemia and normal glucose levels, we did not find statistically significant differences between baseline values and the values obtained during treatment with CSII. We also found no significant changes in the coefficient of variation of blood glucose during the followup (Table 2).
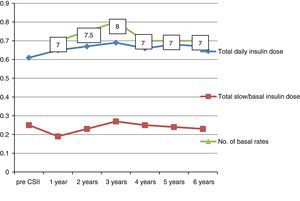
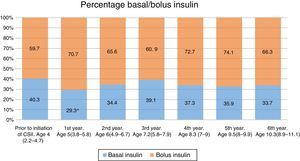
Insulin dosageThe total insulin dose had increased slightly from 0.61 to 0.65IU/kg/day by 1 year of treatment with CSII (Fig. 1). The percentage of basal insulin relative to the total daily dose decreased significantly (from 40% to 29% at 1 year of CSII) (Fig. 2). It increased slightly thereafter.
The number of basal rates per day at 1 year of treatment was 7 (6–8), with no significant changes throughout the followup (Fig. 1). We did not analyse the total number of boluses (mealtime and correction boluses) due to its day-to-day variability.
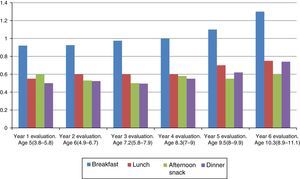
The I/HC ratio was significantly greater for breakfast compared to the insulin requirements for all other meals throughout the followup. Although there is an overall increase in I/HC ratio with age, the increase was more marked for breakfast, with insulin requirements increasing from 0.92IU per carbohydrate serving in the first year of CSII to 1.3IU per carbohydrate serving at 6 years of CSII. The insulin requirements for the afternoon snack remained unchanged throughout the followup (Fig. 3).
Adverse eventsDuring the followup period (median, 5 years [3–6]) there was one episode of severe hypoglycaemia, which occurred in the first year of treatment. The percentage of mildly hypoglycaemic values did not change significantly in the years under study, and ranged between 8.5% and 11% (Table 2).
A single episode of DKA was documented for the entire followup.
The treatment dropout rate was 3.7%. One girl discontinued treatment after 3 years of her own accord. The HbA1c of this patient at the time of discontinuation of CSII was 7.2%, and she had not experienced any episodes of severe hypoglycaemia or DKA.
DiscussionThe use of CSII for the treatment of T1D has increased substantially in the past decade, with evidence of a greater efficacy compared to treatment with MDI. Large international registries such as T1D Exchange Clinic Registry in the United States, the Prospective Diabetes Follow-up Registry (DPV) in Germany and Austria or the National Paediatric Diabetes Audit in the United Kingdom have found a 0.5% reduction in HbA1c in children with T1D treated with CSII.12 However, the mean HbA1c in insulin infusion pump users was 8%. Specifically, the T1D Exchange Clinic Registry assessed children aged less than 6 years, and found a mean HbA1c of 7.9% in children treated with CSII compared to 8.5% in children treated with MDI in this age group.18 In this observational study, only 32% of children treated with CSII achieved HbA1c levels of less than 7.5%, the target recommended by international diabetes societies as a criterion for good metabolic control in the paediatric age group. In our group of preschool-aged children, HbA1c decreased from 6.9% (6.7–7.5%) to 6.8% (6.4–7.1%) in the first year of treatment with CSII, and was maintained at levels below this value through the entire follow-up period. Overall, 95% of children treated with CSII in our study achieved glycaemic control targets during the entire followup.
The success of treatment was mostly due to the diabetes education provided at the Diabetes Unit. The amount of hours invested at treatment initiation and in the subsequent clinical followup is essential in obtaining favourable outcomes. Our CSII education programme consists of four consecutive days of training, and includes learning about all the features in the pump and how to manage them: different types of boluses, bolus calculator, temporary basal rates, alarms, etc. Basal rates and I/HC ratios are adjusted for each individual, systematically and meticulously, based on numerous blood glucose measurements. All families count and weigh carbohydrate servings. The insulin sensitivity index is calculated for the different time intervals, and these data are input in the bolus wizard feature.
One of the main explanations for the outcomes in our study is the daily frequency of CBG tests. Previous studies have already demonstrated that an increased frequency of CBG measurements is associated with a significant improvement in HbA1c. In the DPV study, Ziegler et al.20 found that the level of glycated haemoglobin dropped by 0.2% with each additional glucose measurement. Olsen et al. recently obtained similar results in a study conducted in Denmark.21 Preschoolers in our Diabetes Unit measured their CBG a median of 10 (9–11) times a day in the first year of treatment with CSII, always including a nocturnal measurement between 3:00 and 4:00am. In the last visit for which data were collected, after a median of 5 years of followup of treatment with CSII, we found that patients made a mean 10.5 (9–12) CBG measurements a day. This amounted to 2.5 more measurements a day compared to children of the same age in the DPV study. While measurement of CBG at night may be inconvenient for children and their families, it was nevertheless performed in adherence to the ISPAD guidelines, which recommend measuring blood glucose at bedtime, during the night and upon waking for the main purposes of detecting and preventing low and high glucose levels at night and adjusting basal insulin rates.8 At present, the use of continuous glucose monitoring (CGM) systems facilitates the assessment of nocturnal blood glucose, while the availability of sensor-augmented pumps that suspend insulin delivery in response to predicted low blood glucose can reduce the occurrence of hypoglycaemic episodes, particularly at night.22
One of the main indications for starting treatment with CSII in our preschool-aged patients was the need to reduce glycaemic variability, as an increasing number of studies demonstrate how severe the effects of glucose fluctuations can be in the developing brain.5,7 We used the coefficient of variation to assess glycaemic variability. The current recommendation is to pursue the lowest possible CV.23 Our study did not find changes in the CV in relation to the use of CSII (47% before initiation of CSII; 44.1–48.5% during the years of followup). We believe that while the number of CBG measurements per day was high, it was not sufficient to properly assess changes in CV, and that an accurate estimation would require the use of CGM. We are currently working on this area, trying to assess the changes in the CV in children treated with CSII and under CGM. The situation was similar in the assessment of the percentage of values that were within the normal range, for while we found a significant reduction in HbA1c, we did not find significant changes in the percentage of normal glucose values, although we found an increasing trend that was sustained throughout the followup. We hypothesise that the discrepancy between the statistically significant decrease in HbA1c and the lack of change in the percentages of low, high and normal blood glucose values (Table 2) was due to the fact that the number of CBG measurements in the patients, while high, did not suffice for the appropriate assessment of changes in the percentage of blood glucose levels in or outside the target range, and that CGM would be needed to achieve this research objective.
As described in previous studies,24 the percentage of basal insulin over the total in this age group decreased at the beginning of treatment and progressively increased with the age of the patients.
We were encouraged to find that there was no increase in the incidence of severe hypoglycaemia, independently of HbA1c levels. In the year prior to initiation of CSII, there were three episodes of severe hypoglycaemia: 11.1 episodes/100 patients/year; during the years of followup, the rate decreased to 0.87 episodes/100 patients/year. These findings were very similar to those of a recent study in Slovenia, which reported 1.21 episodes/100 patients/year.25 Two possible explanations for this very low incidence of severe hypoglycaemia are the high number of CBG measurements performed and the meticulous adjustment of basal rates and boluses carried out periodically at the unit and by the parents. Our study clearly showed that insulin requirements vary for the different meals of the day, and that they are greatest at breakfast, which was consistent with previous studies.26
Confirmation of our data requires studies with longer duration of followup and larger samples of preschool children. Studies in children treated with CSII and CGM are also needed for more accurate assessment of glycaemic variability and the frequency of hypoglycaemic and hyperglycaemic events.
To conclude, our experience suggests that long-term treatment with CSII is safe and efficacious in children aged less than 6 years. This study shows that it is possible to achieve and maintain the metabolic goals recommended by international scientific societies.
Conflicts of interestRaquel Barrio has served as a consultant in Novo Nordisk, Lilly and Medtronic advisory boards, and as a speaker in conferences sponsored by Lilly, Novo Nordisk, Roche, Medtronic and LifeScan.
The rest of the authors have no conflicts of interest to declare.
Please cite this article as: Colino E, Martín Frías M, Roldán B, Álvarez MÁ, Yelmo R, Barrio R. Infusión subcutánea continua de insulina en menores de 6 años: evolución a largo plazo. An Pediatr (Barc). 2017;87:276–283.
Previous presentation: this study was presented at the 55th Annual ESPE Meeting; September 10–12, 2016; Paris, France.