Fever is a common cause of paediatric admissions in emergency departments. An aetiological diagnosis is difficult to obtain in those less than 3 months of age, as they tend to have a higher rate of serious bacterial infection (SBI). The aim of this study is to find a predictor index of SBI in children under 3 months old with fever of unknown origin.
MethodsA study was conducted on all children under 3 months of age with fever admitted to hospital, with additional tests being performed according to the clinical protocol. Rochester criteria for identifying febrile infants at low risk for SBI were also analysed.
A predictive model for SBI and positive cultures was designed, including the following variables in the maximum model: C-reactive protein (CRP), procalcitonin (PCT), and meeting not less than four of the Rochester criteria.
ResultsA total of 702 subjects were included, of which 22.64% had an SBI and 20.65% had positive cultures. Children who had SBI and a positive culture showed higher values of white cells, total neutrophils, CRP and PCT. A statistical significance was observed with less than 4 Rochester criteria, CRP and PCT levels, an SBI (area under the curve [AUC] 0.877), or for positive cultures (AUC 0.888). Using regression analysis a predictive index was calculated for SBI or a positive culture, with a sensitivity of 87.7 and 91%, a specificity of 70.1 and 87.7%, an LR+ of 2.93 and 3.62, and a LR− of 0.17 and 0.10, respectively.
ConclusionsThe predictive models are valid and slightly improve the validity of the Rochester criteria for positive culture in children less than 3 months admitted with fever.
La fiebre es motivo frecuente de consulta pediátrica y en menores de 3 meses su diagnóstico etiológico es difícil, siendo un grupo de pacientes con mayor tasa de infección bacteriana grave (IBG). Nuestro objetivo es encontrar un modelo predictivo de IBG en menores de 3 meses con fiebre sin foco.
MétodosSe estudió a los niños menores de 3 meses con fiebre sin foco ingresados, realizándose pruebas complementarias según protocolo clínico. Se analizaron además los criterios de Rochester de bajo grado de IBG. Se diseñó un modelo predictivo de IBG y cultivo positivo, incluyendo las siguientes variables en el modelo máximo: proteína C reactiva (PCR), procalcitonina (PCT) y cumplimiento o no de menos de 4 criterios de Rochester.
ResultadosSe incluyó a 702 sujetos; el 22,64% presentaba IBG y el 20,65% cultivos positivos. Los que presentaban IBG y cultivo positivo presentaron más leucocitos, neutrófilos totales, PCR y PCT. Se obtuvieron significación estadística en puntuación de Rochester menor de 4 y valores de PCR y PCT para IBG (área bajo la curva [ABC] 0,877) y para cultivos positivos (ABC 0,888). Con la regresión se obtuvieron unas fórmulas de predicción de IBG y cultivo positivo con sensibilidad del 87,7 y el 91%, especificidad del 70,1 y el 87,7%, CPP de 2,93 y 3,62 y CPN de 0,17 y 0,10, respectivamente.
ConclusionesLos modelos predictivos son válidos y mejoran discretamente la validez de los criterios de Rochester para cultivo positivo en menores de 3 meses ingresados con fiebre.
Fever is one of the main reasons for paediatric medical visits, accounting for 10–20% of emergency department visits.1
In the field of paediatrics, fever is of greater significance due to the particular characteristics of the patients, the frequent nonspecificity of presenting symptoms in severe infections, and the anxiety that it produces in families and even in health professionals. These factors grow exponentially in young infants, especially those aged less than 3 months—the focus of our study—in who the diagnosis of a potential severe bacterial infection has more serious therapeutic and prognostic implications.
Fever is usually a manifestation of infection, but in infants aged less than 3 months,1 non-infectious aetiologies are more frequent and clinical diagnosis is usually challenging. Fever in these patients tends to be milder, but its presence may be a manifestation of severe disease. We ought to pay particular attention to the subset constituted by newborns (aged less than 4 weeks), who are considered a high-risk group, as up to 1/8 of those presenting with fever may have a severe bacterial infection (SBI). The most frequent causative agents in this age group are β-haemolytic group B streptococci (Streptococcus agalactiae), Enterobacter species (especially Escherichia coli) and Listeria monocytogenes.
The primary objective of our study was to develop an index for the prediction of SBI in infants aged less than 3 months with fever of unknown origin, including clinical features and laboratory tests for acute phase reactants. Furthermore, we sought to establish the ideal cut-off point after which it would be possible to recommend antibiotic treatment fairly reliably and advance the appropriate use of empiric antibiotherapy.
Participants and methodsWe conducted a prospective study of 5 years’ duration in infants aged 0 to 3 months that visited the emergency department with fever of unknown origin of 38°C or higher who, following emergency department protocols based on the recommendations of the Asociación Española de Pediatría (Spanish Association of Paediatrics), underwent microbiological testing and were admitted to hospital. We defined fever without source of short duration as fever whose aetiology remained unknown after a careful history taking and physical examination (PE) and with onset in the past 72h. In adherence with clinical protocols, all patients with a diagnosis of SBI received antibiotic treatment.
A complete blood count with measurement of C-reactive protein (CRP) and procalcitonin (PCT) levels and collection of samples for microbiological testing—blood culture, urine culture (catheter specimen) and stool culture (in case of gastrointestinal manifestations)—were performed in all patients, and analysis of cerebrospinal fluid (following protocol) in 302 patients (43.01%).
We considered the following to be SBIs1: urinary tract infection, occult bacteraemia, meningitis, pneumonia, bacterial gastroenteritis, septic arthritis, osteomyelitis and soft tissue infection.
We also evaluated the original Rochester criteria with the addition of laboratory parameters, assigning one point for each of the laboratory values that were fulfilled and also one point for meeting the criteria established for the anamnesis and the PE.
The Rochester criteria were the following:
- 1.
Temperature≥38°C.
- 2.
Anamnesis:
- a.
Born at term.
- b.
Did not receive perinatal antibiotherapy.
- c.
Has no underlying disease.
- d.
Was not hospitalised longer than the mother.
- a.
- 3.
PE: good general health, with no signs of focal infection.
- 4.
Laboratory values:
- a.
White blood cell (WBC) count: 5000–15,000/mm3.
- b.
Neutrophil count<1500.
- c.
Urine sediment with≤10WBC/hpf.
- a.
We established the following exclusion criteria:
- –
Heart disease.
- –
Primary or secondary immunodeficiency.
- –
Lack of laboratory data (62 patients).
We used SPSS for Windows, version 15.0. We summarised the results of quantitative variables as means and standard deviations, and of qualitative variables as percentages. Differences in quantitative variables were assessed by means of the parametric Student t test or the nonparametric Mann–Whitney test after checking the normality assumption with the Kolmogorov–Smirnov test. We analysed contingency tables for qualitative variables by means of the chi square test. We defined statistical significance as a p-value of less than 0.05.
The association of the variables under study was assessed by means of binary logistic regression, with the dependent variable being the presence or absence of a positive culture, SBI or sepsis-meningitis. We used a backward elimination strategy, starting with a full model that included age, meeting the Rochester criteria, WBC and neutrophil counts and CRP and PCT levels, and then removing independent variables whose coefficient of regression corresponded to a p-value of less than 0.05 while retaining confounding variables in the model. We also analysed ROC curves for the final logistic regression models.
We excluded the presence of collinearity by analysing condition indices and variance inflation factors.
We calculated the sensitivity (Sen), specificity (Spe), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) with their 95% confidence intervals (CIs) using the online software Interactive Statistical Calculation available at http://www.statpages.org/ctab22.html.
ResultsWe included 702 infants, 328 of who were male (46.72%). There were positive culture results in 145 patients; 115 corresponded to urinary tract infections, followed in frequency by sepsis (n=20), meningitis (n=6) and invasive diarrhoea caused by Salmonella (n=3).
Among the SBIs, we also found 21 cases of pneumonia, 13 of cellulitis and 2 of omphalitis.
Following are the percentages of positive cultures, cases of sepsis-meningitis and cases of SBI with the corresponding 95% CIs:
- –
Positive culture: 20.66% (17.82–23.81%).
- –
Sepsis: 2.85% (1.83–4.39%)
- –
Meningitis: 0.85% (0.35–1.90%).
- –
Sepsis-meningitis: 3.70% (2.52–5.39%).
- –
SBI: 22.39% (19.97–26.19%).
The most frequent pathogen was E. coli, detected in 62.75% of cultures (n=91, 6 as the aetiological agent in meningitis) and 73.9% of urinary infections (n=85), followed by Enterococcus species, found in 15 patients. S. agalactiae was found in 35% of sepsis cases (n=7) and 20% of meningitis cases (n=1). Streptococcus pneumoniae accounted for 25% of sepsis cases (n=5) and 40% of meningitis cases (n=2).
Table 1 presents data for epidemiological variables and the documented blood test values.
Table 2 presents the age and laboratory parameters by positive or negative result of culture, with a statistically significant increase in laboratory values associated with the former (P<.0001).
Age and laboratory parameters by result of culture: mean (standard deviation).
| Positive culture | Negative culture | Significance | |
|---|---|---|---|
| Age (months) | 1.40 (0.83) | 1.31 (0.74) | NS |
| WBC | 15584 (6.593) | 12356 (6409) | P<.001 |
| Neutrophils | 7664 (3753) | 5447 (4174) | P<.001 |
| CRP (mg/dL) | 4.82 (5.56) | 2.06 (2.96) | P<.001 |
| PCT (ng/mL) | 2.47 (7.59) | 0.57 (2.09) | P<.001 |
Table 3 shows an equivalent comparison based on the presence or absence of SBI, with similar results.
Age and laboratory values by presence of SBI: mean (standard deviation).
| SBI | No SBI | Significance | |
|---|---|---|---|
| Age (months) | 1.44 (0.80) | 1.29 (0.74) | .046 |
| WBC | 15982 (6980) | 11996 (6103) | P<.001 |
| Neutrophils | 8139 (5190) | 5130 (3646) | P<.001 |
| CRP (mg/dL) | 4.96 (5.40) | 1.83 (2.68) | P<.001 |
| PCT (ng/mL) | 2.23 (6.92) | 0.52 (2.04) | P<.001 |
Table 4 presents the results of binary logistic regression for positive culture, SBI or diagnosis of sepsis and/or meningitis, which were statistically significant for the following: CRP and PCT, laboratory parameters, and a score of less than 4 in the Rochester criteria. The model was less reliable for sepsis and/or meningitis, with a noticeably smaller area under the curve (AUC) and larger p-values for sepsis and/or meningitis.
Results of binary logistic regression: coefficient (confidence interval).
| Positive culture | SBI | Sepsis or meningitis | |
|---|---|---|---|
| CRP (mg/dL) | 1.058 (1–1.118) P=.049 | 1.124 (1.047–1.207) P=.001 | 1.175 (1.079–1.216) P<.001 |
| PCT (ng/mL) | 1.087 (0.996–1.187) P=.063 | 1.128 (1.015–1.254) P=.025 | 1.106 (0.994–1.118) P=.066 |
| Rochester < 4 | 36.097 (18.58–70.09) P<.001 | 67.701 (33.41–137.15) P<.001 | 36.09 (18.58–70.09) P=.01 |
| R2 of the model | 0.505 | 0.632 | 0.234 |
| Significance | P<.001 | P<.001 | P=.005 |
| Area under t | 0.888 (0.859–0.916) | 0.877 (0.850–0.904) | 0.650 (0.573–0.727) |
pC+=0.024+CRP×1.058+PCT×1.087+36.1 (R<4).
pSBI=0.017+CRP×1.124+PCT×1.128+67.701 (R<4).
When it came to the Rochester criteria, we obtained an AUC for SBI (corresponding to not meeting the criteria) of 0.69 (0.573–0.809), with a Sen of 0.917 (0.870–0.950), a Spe of 0.820 (0.804–0.831) and a LR− of 0.966 (0.947–0.980) for meeting fewer than 4 criteria.
As supplementary information, we present the Sen and Spe for other cut-off points with their 95% confidence intervals in the predictive model for SBI:
SBI 50: Sen, 0.75 (0.68–0.80); Spe, 0.66 (0.64–0.68)
SBI 75: Sen, 0.86 (0.80–0.90); Spe, 0.68 (0.66–0.69)
Thus, the model can be summarised into the following probability formulas:
- 1.
“The probability of obtaining a positive culture (pC+) is obtained by adding a constant (0.024), to the obtained CRP value×1.058, the obtained PCT value×1.087, and 36.097 if the score for the Rochester criteria is <4″ (pC+=0.024+CRP×1.058+PCT×1087+36.1 [R<4]).
- 2.
“The probability of having a SBI is obtained by adding a constant (0.017) to the obtained CRP value×1.124, the obtained PCT value×1.128, and 67.701 if the score for the Rochester criteria is <4″ (pSBI=0.017+CRP×1.124+PCT×1.128+67.701 (R<4]).
Table 5 presents the cut-off points for the SBI and positive culture models, with a LR− of 0.10 and 0.17 for the cut-off points of 80 and 25, respectively, which indicate that there is a very low probability of obtaining a false negative (FN) with these models. The NPVs for SBI and positive culture were 0.982 (0.964–0.992) and 0.987 (0.970–0.995), respectively.
Value of diagnostic tests for the positive culture and SBI models, with their corresponding cut-off points.
| Positive culture | SBI | |
|---|---|---|
| Cut-off point | 25 | 80 |
| Sensitivity | 91.9 (85–95) | 87.7 (80–92) |
| Specificity | 74.7 (73–75) | 70.1 (68–71) |
| OR | 33.29 (16.34–69.76) | 16.70 (9.15–30.93) |
| Positive likelihood ratio | 3.62 (3.19–3.92) | 2.93 (2.56–3.21) |
| Negative likelihood ratio | 0.10 (0.05–0.19) | 0.17 (0.10–0.20) |
Comparison of the models for SBI and positive culture: statistics.
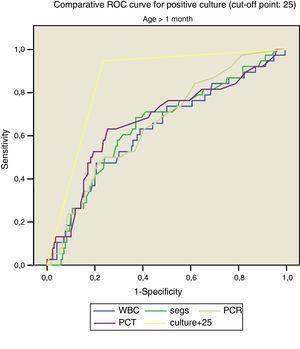
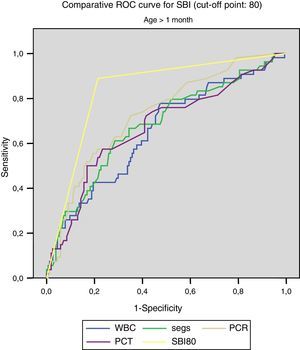
Figs. 1 and 2 compare our model with the obtained laboratory values, and we did not find statistically significant differences in the model when we divided the sample into newborns and infants aged more than 1 month.
Thus, for positive culture (cut-off point, 25):
- –
<1month: AUC, 0.862, with a standard error of 0.041; 95% CI, 0.782–0.943.
- –
>1month: AUC: 0.832, with a standard error of 0.043; 95% CI, 0.798–0.914.
For SBI (cut-off point, 80):
- –
<1month: AUC, 0.832, with a standard error of 0.043; 95% CI, 0.747–0.916.
- –
>1month: AUC, 0.837, with a standard error of 0.032; 95% CI, 0.775–0.899.
Using this model, 56.26% of the patients in the sample would not receive unnecessary antibiotic treatment applying the established threshold for SBI, and 66.52% would not receive it applying the threshold for positive culture.
DiscussionIn our sample, we found a prevalence of SBI of 22.64%, with a proportion of positive cultures of 20.65%; furthermore, the laboratory parameters showed significantly higher WBC and total neutrophil counts and CRP and PCT values in children with positive cultures and SBI.
A systematic review of 14 studies2 found a mean prevalence of 20.5% (4.5–29.3%).
Analysing the laboratory parameters, we found that patients with SBI in our study (n=180) had a mean WBC count of more than 15000 (15982), a slightly lower mean neutrophil count (8139), CRP values of approximately 5mg/dL (4.96) and PCT values greater than 2ng/mL (2.23). We ought to remember that the AUC for the SBI model ranged between 0.66 for the WBC count and 0.73 for CRP.
Other studies, such as the one by Cuello García et al.,3 with AUCs of 0.6 for neutrophils, 0.55 for the total WBC count and 0.71 for CRP, report values slightly below those obtained in our study. Their study did not analyse the levels of PCT. Yet others have found AUC values greater than those found in our study, such as that by Fernández López4 et al. from 2003, with AUCs of 0.95 for PCT and 0.81 for CRP, or the study of Andreola et al.,5 with AUCs of 0.82, 0.85, 0.81 and 0.74 for PCT, CRP, total WBC count and neutrophils. Another study published in 2008 by Maniaci et al.6 reported an AUC of 0.66 for the WBC count (the same as ours) and AUCs of 0.74 for the neutrophil count and 0.82 for PCT, greater than those obtained in our study.
A validation study of a laboratory risk index score carried out by Galetto-Lacour et al. in 20107 found that the score varied with PCT values above or below 0.5 and 2, CRP values above or below 40 and 100, and positive versus negative urine dipstick results. This study (n=106 infants aged less than 3 months) obtained an AUC of 0.91 for the index score, which was greater than the AUCs obtained for PCT, CRP or WBC individually, which were of 0.84, 0.86 and 0.71, respectively.
This last study, which is of great relevance, suggested that the combination of parameters achieved a greater diagnostic accuracy.
Analysing the Rochester criteria in our sample, we found that 165 patients out of the 180 in whom a SBI was detected did not meet the low risk criteria, with the sensitivity, specificity, NPV and AUC reported above. When we analysed the sepsis-meningitis subset separately, we obtained a Sen of 63.63%, a Spe of 63.97% and a NPV of 98.19%. These figures, which were quite good for SBI overall, could certainly be improved in this subset, especially when it comes to sensitivity for ruling out sepsis-meningitis. The low number of these patients in our sample may explain the inaccuracy of the data. Thus, in the low-risk group, the risk of having a SBI (FN) was 2.13% and the risk of having sepsis-meningitis was 1.80%, whereas in the high-risk group, the risk of presenting a SBI (true positive) was 65.63% and the risk of having sepsis-meningitis was 5.4%.
Our model did not exclude any patients with SBI, perhaps at the price of a decreased specificity, however, the model is enriched by taking into account laboratory parameters that were not originally included in the Rochester criteria.
Our findings diverge from those of other published studies, the classic one being that by Baraff et al.,8 which found a similar risk of SBI and sepsis-meningitis in the low-risk group (2.6% and 1.3%), but a much lower risk of SBI (24.3%) in the high-risk group, approximately 40% smaller than the risk we found. In different prospective studies that used these criteria, alone or combined with the Philadelphia criteria, the prevalence of SBI in the high-risk group ranged from 12.3% to 33.9% of patients,9–12 which also stood in contrast to our percentage of 65.63%. These same studies, however, have reported similar risk percentages in the low-risk group, with a variability that ranged between 0% and 5.97%. A literature review from 2010 that analysed all the studies published on the subject, a total of 21,13 concluded that the estimated risk of SBI in the high-risk group ranged between 19.8% and 23.8% (in prospective versus retrospective studies), while it ranged between 0.67% and 2.71% in the low-risk group. These data are largely consistent with the low-risk group in our study, but not the high-risk group. This discrepancy may be explained by the populations under study, as our sample consisted of hospitalised patients, whereas these other studies were conducted in the general population.
In our multivariate analysis, only the CRP and PCT values and a score of less than 4 for the Rochester criteria remained in the models for the presence of positive culture, SBI or diagnosis of sepsis and/or meningitis, although in the latter model both the R2 (0.234) and the AUC (0.650) were mediocre. Using the results obtained through logistic regression, we calculated the Sen, Spe, odds ratio (OR), LR+ and LR− for the cut-off points of 25 (for positive culture) and 80 (for SBI).
There have been several previous “attempts” to establish a predictive model for SBI: we ought to mention a study published in 1991 by Downs et al.14 in children aged 2–24 months which, based on the clinical status of the patient and the WBC count (greater or less than 15000 cells per field), concluded that the best approach to the management of febrile infants considered at risk was empiric antibiotherapy in all. It is worth noting that while this study mentioned factors such as the erythrocyte sedimentation rate or the level of CRP it did not take them into account, and that patients without manifestations of meningitis were excluded from the analysis. Along the same lines, in a review published 12 years later in the British Medical Journal,15 Brook advocated a conservative approach to the management of febrile infants aged less than 3 months, proposing that they be admitted to hospital and be given empiric antibiotic treatment pending the results of bacterial culture, although the author already remarked on the disadvantages of hospitalisation, untoward comorbidity and side effects of antibiotherapy that may result from this approach. One year later, the American Medical Association16 published a study conducted in 3066 febrile infants aged less than 3 months, a fairly large sample, analysing the role of clinical scales and the WBC count in the management of these patients. Unlike our study, it included patients that had been discharged from hospital, and concluded that the parameters associated most strongly to SBI were age and clinical appearance, without taking into account CRP or PCT levels. Continuing our review of models published in the literature that preceded the one presented here, we should probably comment on the one that has the most similarities with ours, which corresponds to the study by Bachur and Harper17 published in 2001. This study included in the analysis 5279 infants aged less than 3 months that sought emergency care for fever. It did also not take into account CRP or PCT values, but it did consider the WBC count and results of urine analysis. The proportion of SBI that they reported was considerably lower compared to ours: 7% compared to 22.64%, the prevalence calculated in our series, which was similar to the prevalence found in the aforementioned study by Brook, although it was not specified whether cases of pneumonia were included in the analysis. The pathogen detected most frequently was group B streptococcus, contrary to our series, in which E. coli was most frequent; their sensitivity was slightly lower than ours (82% vs 87%), while they obtained a higher specificity (76% vs 68.3%), which probably has to do with the cut-off point that we established. Their reported OR of 12 was also lower than the one we obtained, which was 14.41.
Our model also achieved a higher diagnostic accuracy than that achieved with the individual use of WBC count, neutrophil count, CRP or PCT. We did not find differences in the applicability of the model to newborns versus infants aged more than 1 month, and we could establish with a high degree of certainty the probability of obtaining positive culture results in our patients (except in cases of nonbacteraemic pneumonia) through the application of the alternative formula for positive culture.
The high NPV and the low negative likelihood ratios (0.10 and 0.17, respectively, see Table 5), along with the high impact of this model in our sample, provide reasonable grounds to decide against initiating antibiotic treatment in patients identified as being at low risk, although this requires subsequent validation. Furthermore, our model would only fail to classify 0.99% of patients with SBI and 0.71% of patients with positive cultures correctly.
We expect and hope that this model will be useful in everyday clinical practice and as a basis for future studies.
ConclusionsAfter conducting this study, we can reasonably conclude that it is possible to develop a predictive index for SBI (SBIPI) and for positive culture results (PCPI) for infants aged less than 3 months with fever of unknown origin integrating clinical and laboratory findings. Both indices were able to reliably diagnose most cases of SBI in our sample with the established cut-off points, with a LR− very close to zero.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villalobos Pinto E, Sánchez-Bayle M. Creación de un modelo probabilístico de diagnóstico de infección bacteriana grave en lactantes febriles de 0 a 3 meses de vida. An Pediatr (Barc). 2017;87:330–336.