Congenital diaphragmatic hernia (CDH) remains a therapeutic challenge. The surgical classification recommended by the Congenital Diaphragmatic Hernia study group (CDHSG), based on the size of the defect, is used for staging in reference centres. Larger defects are associated with poorer outcomes. Our aim was to describe and compare the morbidity at hospital discharge of newborns who underwent surgical correction of CDH at the Juan P. Garrahan, according to the surgical staging of the defect proposed by the CDHSG.
Material and methodsThe study included patients with CDH admitted to the Juan P. Garrahan Hospital between 2012 and 2020, and we analysed the distribution, morbidity and mortality associated with the size of the defect. We carried out a descriptive analysis, calculating measures of central tendency and dispersion, and bivariate and multivariate analyses.
ResultsA total of 230 patients with CDH were admitted and 158 underwent surgery. We found that defect sizes C and D sizes were associated with an increased risk of chronic pulmonary disease (CPD) (OR, 5.3; 95% CI, 2.2–13.4; P<.0000), need of extracorporeal membrane oxygenation (OR 3.9; 95% CI, 1.3–12.8; P<.005) and chylothorax (OR, 2.1; 95% CI, 0.8–6.4; P<.10]. The multivariate analysis revealed that a large defect size (C–D) was independently and significantly associated with CPD (OR 4.19; 95% CI, 1.76–9.95).
ConclusionStaging the defect according to de CDHSG classification during surgery allows the application of uniform management criteria and the prediction of patient outcomes and complications during the hospital stay.
La Hernia Diafragmática Congénita (HDC) continúa siendo un desafío terapéutico en el periodo neonatal. La clasificación quirúrgica según el tamaño del defecto, recomendado por el Congenital Diaphragmatic Hernia Study Group (CDHSG), se utiliza para estadificar en los centros de referencia. Los defectos de mayor tamaño se asocian con peores resultados. El objetivo de este estudio, es describir y comparar la morbilidad al egreso hospitalario de recién nacidos operados de HDC, según la estadificación quirúrgica del defecto propuesta por el CDHSG.
Material y métodosSe seleccionó una población de pacientes con HDC que ingresaron al Hospital de Pediatría Juan P. Garrahan entre el año 2012 y 2020 y se analizó la distribución, morbilidad y mortalidad asociadas al tamaño del defecto. Se realizó un análisis descriptivo, con medidas de tendencia central y dispersión, análisis bivariado y multivariado.
ResultadosIngresaron 230 pacientes con diagnóstico de HDC, 158 pacientes fueron operados. Se evidenció que los defectos clasificados como C y D presentaron mayor riesgo de Enfermedad Pulmonar Crónica (EPC) [OR 5.3 (IC 95% 2.2–13.4) p<0.0000], mayor requerimiento de ECMO [OR 3.9 (IC 95% 1.3–12.8) p<0.005] y aumento en la incidencia de quilotórax [OR 2.1 (IC 95% 0.8–6.4), p<0.10], con respecto a los defectos de menor tamaño, A y B. En el análisis multivariado, se observó que los defectos de mayor tamaño (C–D), se asocian con EPC de forma independiente y estadísticamente significativa [OR 4.19 (IC 95% 1.76–9.95)].
ConclusionesEstadificar el tamaño del defecto, según la clasificación del CDHSG en el acto quirúrgico, unifica criterios de atención y predice la evolución y complicaciones de estos pacientes durante la internación.
Congenital diaphragmatic hernia (CDH) is an anomaly characterised by a defect in the diaphragm leading to protrusion of abdominal organs into the thoracic cavity and pulmonary hypoplasia. Its approximate incidence is of 1 per 3000 live births. In 85% of cases, the defect is on the left side, in 13% on the right side and only in 2% of cases bilateral. The upward protrusion of abdominal contents into the thoracic cavity causes compression of the lungs and heart, which complicates the development of these organs. The presence of pulmonary hypertension, resulting from the decreased pulmonary vascular bed secondary to pulmonary hypoplasia, causes severe haemodynamic complications and is associated with a high morbidity and mortality.1–6
Survival is variable and depends on patient-related factors, the severity of pulmonary hypoplasia, the presence of additional malformations, prematurity, the Apgar score or the presence of genetic syndromes.7–10 Some of the factors that affect outcomes in these patients are an appropriate perinatal care and management in a facility with the necessary expertise and resources for the level of complexity that can provide high-quality care.9,11
Large defects are associated with an increased mortality compared to small defects. The morbidity and complications observed in patients that survive surgery are heterogeneous. Their association with defect size continues to be an area of interest in this complex condition.12,13
The aim of our study was to describe and compare the morbidity at discharge in infants operated for CDH in the Hospital de Pediatría Juan P. Garrahan (a children’s hospital) based on the surgical defect staging system proposed by the Congenital Diaphragmatic Hernia Study Group (CDHSG).
MethodsWe conducted a retrospective, descriptive and inferential study. The sample included all patients operated for correction of CDH admitted to the department of neonatal intensive care of the Hospital de Pediatría Juan P. Garrahan between January 1, 2012 and December 31, 2020.
The Department of Neonatal Intensive Care of the Hospital de Pediatría Juan P. Garrahan is a level IIIb reference unit for newborns with CDH. Since 2012, it provides data to the CDHSG. It admits between 25 and 30 patients a year, some of whom are referred from other facilities.14
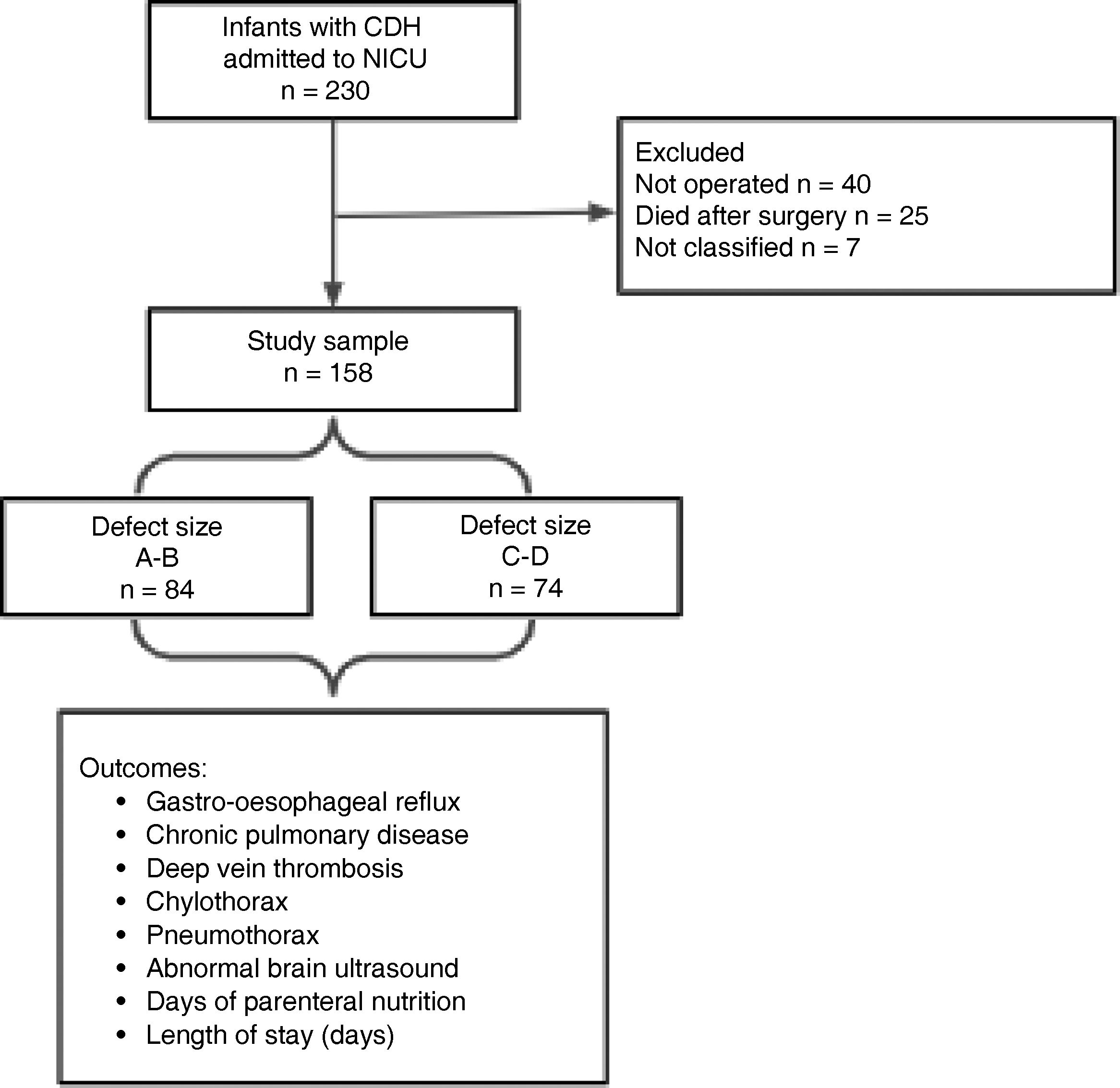
We excluded patients who were not operated, operated patients who died before discharge and operated patients who were not classified according to the CDHSG staging system.
The CDHSG is a collaborative, multicentre register of patients with CHD.15 This group proposed a staging system based on the size of the defect observed by the surgeon during the procedure, classifying defects as type A, B, C or D, which reflect increasing size, based on direct observation. Type A defects are the smallest, with more than 90% of the diaphragm tissue present and involving less than 10% of the chest wall. Type B defects involve at least 50% of the chest wall with absence of 50%–75% of the diaphragm tissue. Type C defects involve more than 50% of the chest wall with absence of more than 50% of diaphragm tissue. Type D defects are the largest, involving more than 90% of the chest wall with less than 10% of the normal diaphragm tissue.16
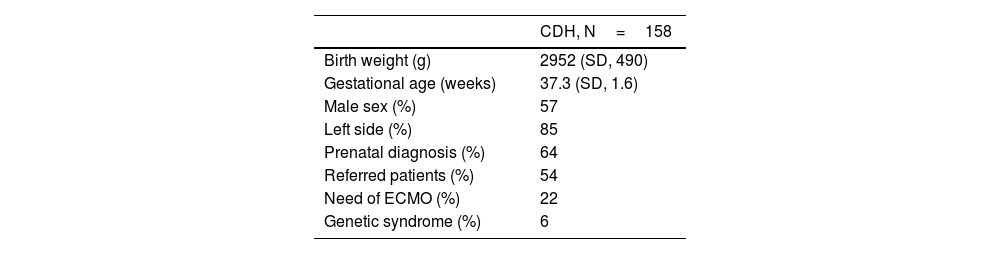
The descriptive variables were the birth weight (BW), gestational age, sex, side of the defect (right/left), prenatal/postnatal diagnosis, referral from another centre, presence of a genetic syndrome or associated malformations.
The outcome variables were the presence of chronic pulmonary disease (CPD), defined as need of respiratory support for more than 30 days, the duration (days) of parenteral nutrition (PN), the length of stay (days) and the need of extracorporeal membrane oxygenation (ECMO). We analysed the presence of complications, such as deep vein thrombosis (DVT), diagnosed by means of Doppler ultrasound, gastro-oesophageal reflux (GOR) defined based on clinical or radiological features, pneumothorax diagnosed by imaging, chylothorax diagnosed based on the presence of chyle in the pleural space requiring drainage and dietary modification, and the presence of pathological lesions on the brain ultrasound. Patients were followed up through discharge from hospital.
In the descriptive statistical analysis, we calculated measures of central tendency and dispersion for quantitative variables and percentages for categorical variables. We used the Wilcoxon test to compare numerical variables, the Kruskal–Wallis test to compare multiple variables and the χ2 test to compare categorical variables. We established a dichotomous classification of the sample by defect size: small (types A–B) and large (types C–D). We performed a bivariate analysis and multivariate analysis with multiple logistic regression fitted with the Hosmer-Lemeshow test. We considered P values of less than 0.05 statistically significant. The statistical analysis was performed with the software STATA SE 12 (StataCorp LP, USA). The study was approved by the Board of Education and Research and the Ethics Committee of the Hospital de Pediatría Juan P. Garrahan.
ResultsIn the period under study, a total of 230 patients with a diagnosis of CDH were admitted to the Department of Neonatal Intensive Care of the Hospital de Pediatría Juan P. Garrahan. We excluded 72 patients, of who 40 died before surgery, 25 following surgical repair, and 7 who were operated but for whom the CDHSG stage had not been documented. Thus, the analysis included 158 patients (Fig. 1).
Table 1 summarises the demographic characteristics of the patients.
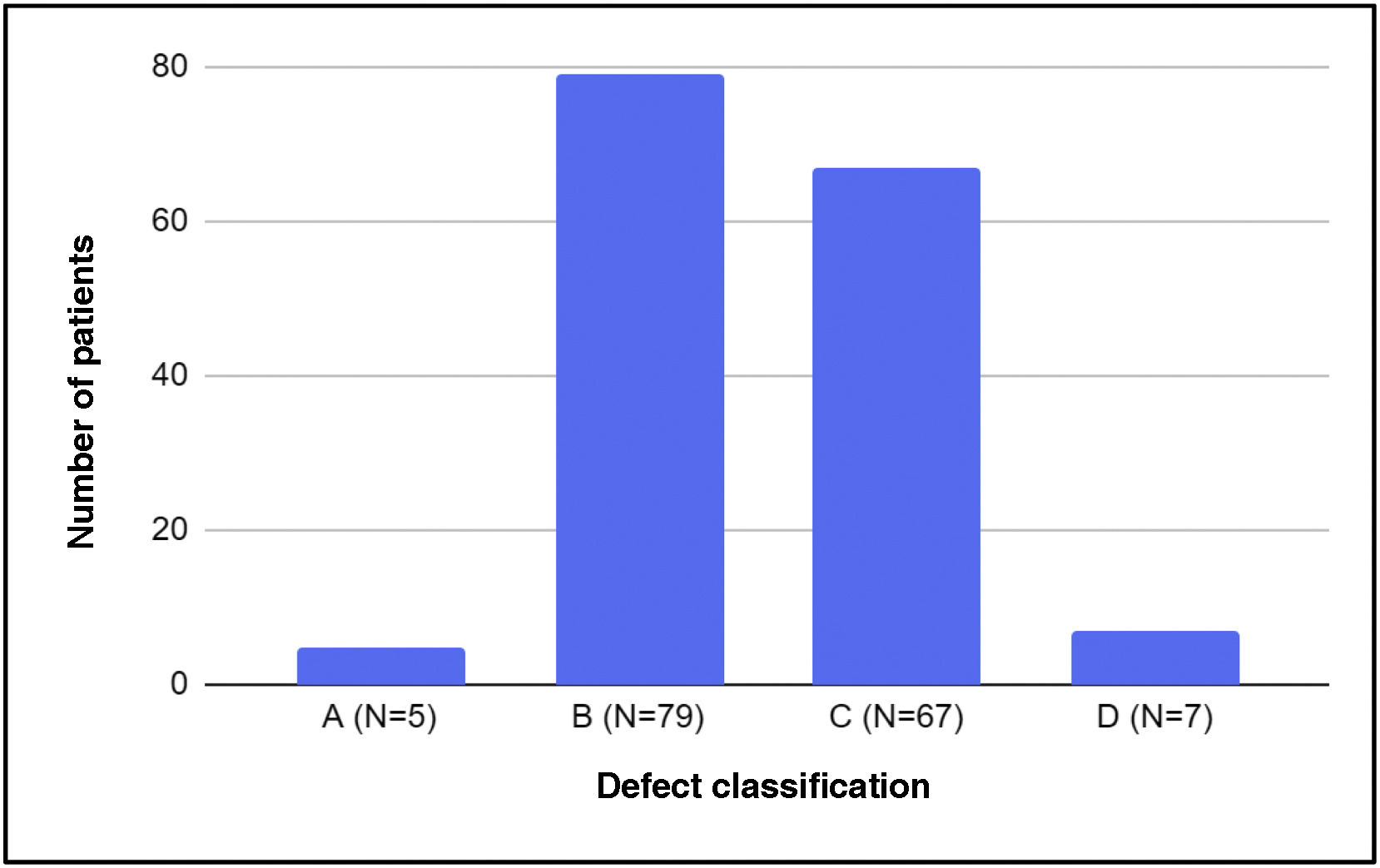
Based on the CDHSG staging system, we found that the most frequent types of defect were B and C, and defects with extreme sizes (A or D) amounted to less than 10% of the sample (Fig. 2)
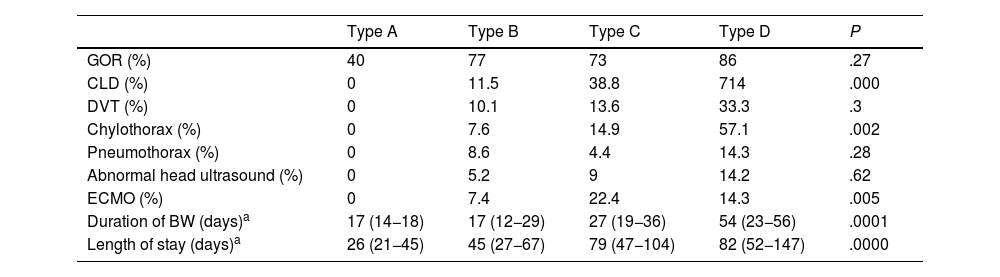
Table 2 presents the results for the variables under study based on the size of the defect.
Comparison of outcomes based on size defect.
| Type A | Type B | Type C | Type D | P | |
|---|---|---|---|---|---|
| GOR (%) | 40 | 77 | 73 | 86 | .27 |
| CLD (%) | 0 | 11.5 | 38.8 | 714 | .000 |
| DVT (%) | 0 | 10.1 | 13.6 | 33.3 | .3 |
| Chylothorax (%) | 0 | 7.6 | 14.9 | 57.1 | .002 |
| Pneumothorax (%) | 0 | 8.6 | 4.4 | 14.3 | .28 |
| Abnormal head ultrasound (%) | 0 | 5.2 | 9 | 14.2 | .62 |
| ECMO (%) | 0 | 7.4 | 22.4 | 14.3 | .005 |
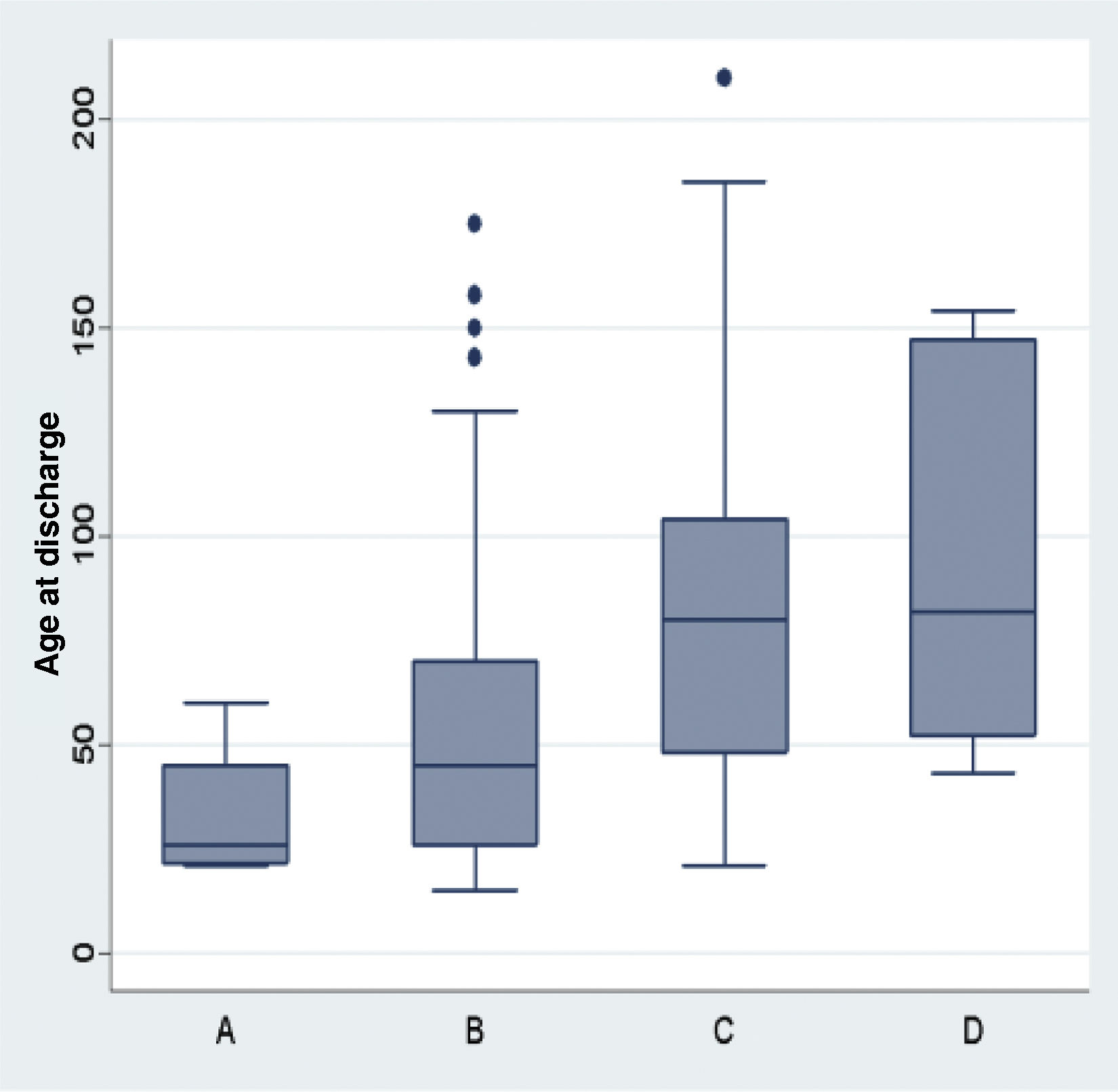
| Duration of BW (days)a | 17 (14−18) | 17 (12−29) | 27 (19−36) | 54 (23−56) | .0001 |
| Length of stay (days)a | 26 (21−45) | 45 (27−67) | 79 (47−104) | 82 (52−147) | .0000 |
CLD, chronic lung disease; DVT, deep vein thrombosis; ECMO, extracorporeal membrane oxygenation; GOR, gastro-oesophageal reflux; BW, parenteral nutrition.
We were only able to analyse the association of mortality with defect size in 25 patients, who died after surgery. Of these patients, 95% had defects graded as C or D.
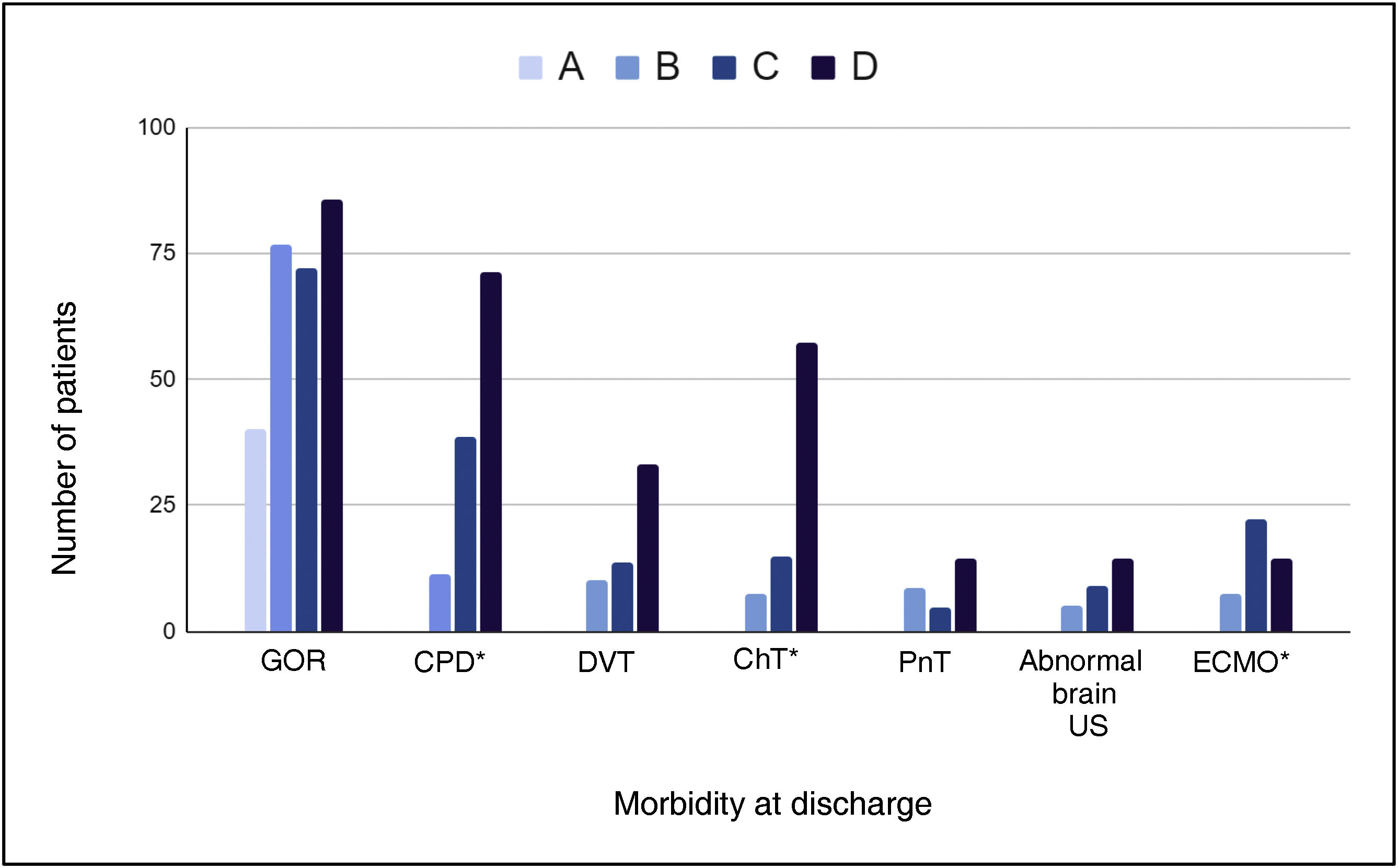
We analysed the morbidity that developed during the hospital stay in relation to the size of the defect. We found a significant association between defect size and CPD, chylothorax and the need of ECMO (Fig. 3).
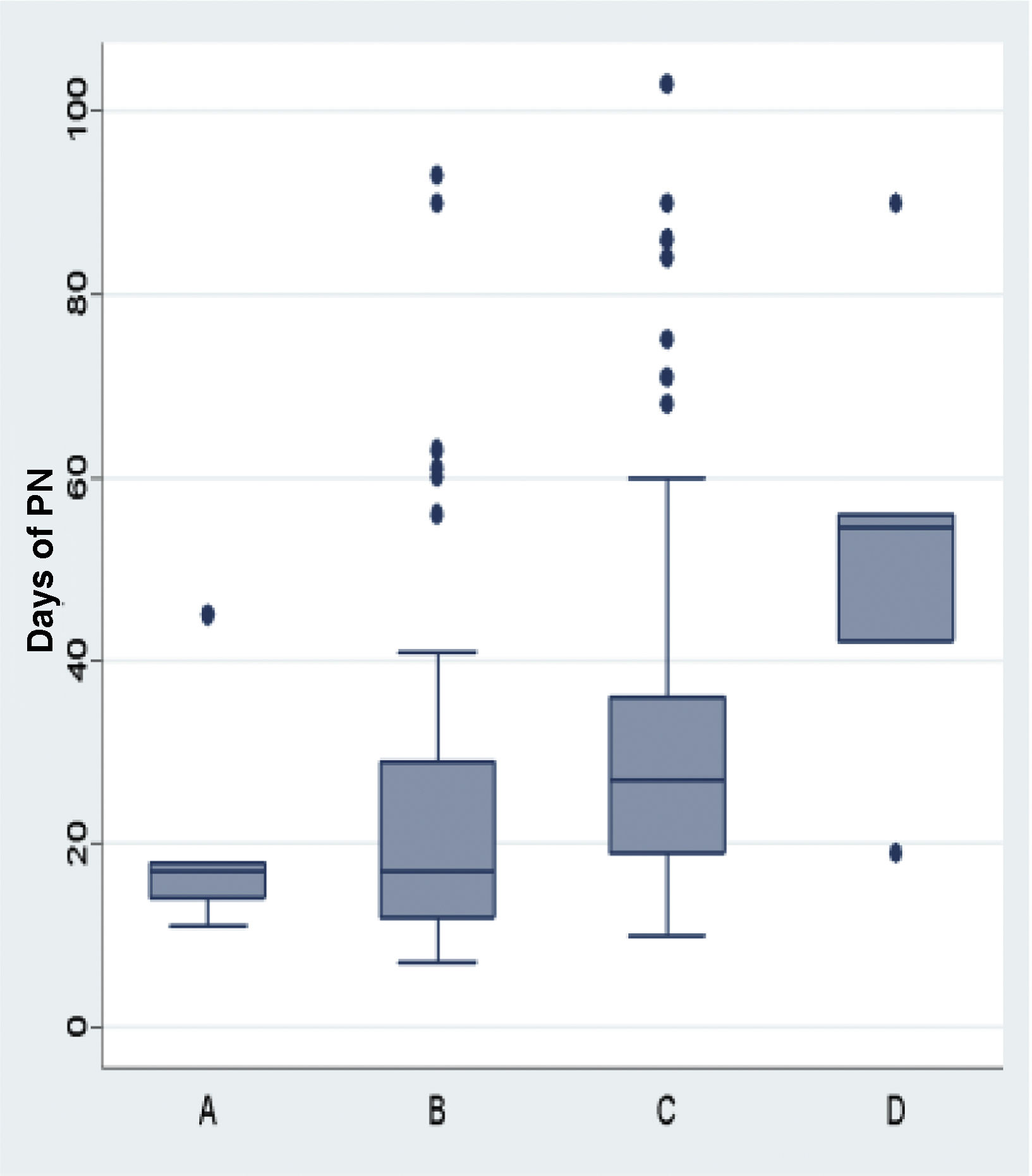
The size of the defect was also associated with the length of stay and the duration of PN in days (Figs. 4 and 5).
The remaining outcomes (GOR, DVT, pneumothorax and pathological brain ultrasound) were not significantly associated with defect size.
In the bivariate analysis, we found that defect types C and D were associated with an increased risk of CPD (odds ratio [OR], 5.3; 95% CI, 2.2–13,4; P<.0000), needing ECMO (OR, 3.9; 95% CI, 1.3–12.8; P<.005) and chylothorax (OR, 2.1; 95% CI, 0.8–6.4; P<.10).
In the multivariate analysis, including the variables CPD, ECMO and chylothorax in the model, we found that large defects (C or D) were independently and significantly associated with CPD (OR, 4.19; 95% CI, 1.76–9.95). The model exhibited an adequate fit and discrimination (Hosmer–Lemeshow test: P=0.1).
DiscussionCongenital diaphragmatic hernia continues to pose a therapeutic challenge. In recent years, the increase in survival of patients with this disease has been associated with an increase in long-term morbidity, including undernutrition, chronic pulmonary disease and neurodevelopmental disorders, conditions that have an impact on quality of life.17 Our study found an association between defect size and morbidity in the short and medium term. The larger the defect, the greater the probability of chylothorax, needing ECMO and CPD. This association was found irrespective of where the defect was located (left or right side). This was consistent with the reports of other authors, who found that the morbidity associated with large defects was not affected by laterality.18
The staging system proposed by the CDHSG is an anatomical classification that allows the homogenization of surgical severity criteria and is strongly associated with mortality.12 Lally et al. reported that applying this staging system with 4 categories (A, B, C and D), subdivided based on whether or not there is also a congenital heart defect, each category is associated with survival rates that go from 99% to 39%, in decreasing order, and a survival of 0% in patients who do not undergo surgical repair. The authors concluded that the purpose of staging the defect is simply to allow standardised reporting, and that this classification should not be used as a prognostic factor.16 In our study, the association with congenital heart defects was infrequent, as only 2 of the patients had a congenital heart defect requiring surgical repair, both of who died before discharge and therefore were excluded from the analysis.
Morini et al. observed that defect size correlated to the magnitude of developmental defects in the patient, such as the presence of the liver in the chest, cardiac anomalies and other associated malformations, and concluded that defect size could be a marker of the severity of developmental abnormality, explaining its association with patient outcomes.19
A systematic review found that pulmonary complications were more frequent in patients who underwent patch repair of the hernia or who had been admitted on ECMO. Patients with patch repairs are often the patients with the largest defects, which is why patch repair could serve as a surrogate marker of severity.20 Defect size has been found to be strongly associated with specific forms of morbidity. Guglielmetti et al. found that defect size was the strongest predictor of requiring an oesophageal fundoplication (Nissen fundoplication) due to severe GOR in the multivariate analysis (OR, 6.93; 95% CI, 3.17–17.07; P<.0001). We did not observe this association in our study. Although the incidence of GOR was similar in our sample, none of the patients underwent fundoplication in the period under study, and they were instead managed with pharmacotherapy.21 Putnam et al., in a cohort of 3665 patients registered in the CDHSG database, found an association between defect size and length of stay (type A defect A, 22 days vs. type D defect, 89 days) and a greater probability of respiratory, gastrointestinal and neurologic morbidity. The multivariate analysis showed that defect size was the strongest predictor of morbidity at discharge. The authors also conducted a longitudinal analysis between 2007 and 2014, reporting that morbidity had improved in patients with small defects but remained stable in patients with larger defects. Some of our findings were consistent with those of Putnam et al; for instance, respiratory morbidity at discharge was the variable associated most strongly to defect size in the multivariate analysis. In our study, we did not find a statistically significant association with any of the other variables, probably due to the sample size.13
When we analysed the distribution of patients in our hospital by defect size, we found differences compared to the CDHSG report. For instance, in our hospital type D defects amounted to 5% of the total, whereas in the CDHSG study they amounted to 13%. The same occurred with type C defects, which corresponded to 37% of cases in our study versus 30% in the CDHSG study. This could be attributed to the subjectivity of the rater, as in our hospital only complete diaphragm agenesis is considered a type D defect.16,22
Among the strengths of the study, we may mention that, since it was conducted in a single centre, the therapeutic approach remained homogeneous throughout the follow-up, following the same protocol in all cases, and that a single surgical team determined the defect size.
Among the limitations, we should highlight that we only analysed morbidity at the time of discharge, without considering the development of additional morbidity in the long term. We ought to mention that all of these patients are assessed during childhood by the follow-up care team of our hospital.
ConclusionIn our study, defect size was significantly associated with the presence of CPD, the need of ECMO, chylothorax, the length of stay in days and the days of PN.
Grading the defect size using the CDHSG staging system helps homogenize care delivery and predict patient outcomes during the hospital stay. The long-term follow-up and outcomes in these patients will provide evidence on the predictive value of defect size.
FundingThis research did not receive any external funding.
Conflicts of interestThe authors have no conflicts of interest to declare.