In this article we present a protocol for the use of the low-FODMAP diet in paediatric patients and review of the current evidence on its efficacy. These short-chain carbohydrates, which can be fermented by the intestinal microbiota, are found in a wide variety of foods, mainly of plant origin. The low-FODMAP diet is a therapeutic tool used for the management of gastrointestinal disorders such as irritable bowel syndrome. The sources we used were PubMed, Web of Science, Google Scholar and institutional websites. Following consumption of FODMAP-rich foods, a series of end products are generated that are not absorbed, giving rise to symptoms. Before starting a low-FODMAP diet, it is important to carry out a diagnostic evaluation including any applicable tests. Treatment is structured in 3 phases: elimination, reintroduction and personalization phase. In the first phase, FODMAP-rich foods are eliminated for 2–3 weeks. In the second phase, lasting 8 weeks, FODMAP-rich foods are gradually reintroduced. The last phase consists in customizing the diet according to individual tolerance. This article details which foods contain FODMAPs and possible substitutes. In addition, specific food diary/intake tracking and educational materials are provided in a series of appendices to facilitate adherence to the diet. Although most studies have been conducted in adults, there is also some evidence on the beneficial effects in the paediatric age group, with a reduction of symptoms, especially in patients with functional gastrointestinal disorders. Nevertheless, more research is required on the subject.
En este artículo presentamos un protocolo para el uso de la dieta baja en FODMAPs en pacientes pediátricos y una revisión sobre la evidencia actual de su eficacia. Estos hidratos de carbono de cadena corta, fermentables por el microbiota intestinal, se encuentran fundamentalmente en gran variedad de alimentos de origen vegetal. La dieta baja en FODMAPs es una herramienta terapéutica utilizada en trastornos digestivos como el síndrome del intestino irritable. Los recursos utilizados han sido PubMed, Web of Science, Google Scholar y páginas web oficiales. Tras el consumo de alimentos ricos en FODMAPs, se generan unos productos finales que no se absorben, causando sintomatología. Antes de comenzar dicha dieta, es importante realizar una aproximación diagnóstica a través de las pruebas complementarias pertinentes. El tratamiento se divide en 3 fases: eliminación, reintroducción y personalización. En la primera se eliminan los alimentos ricos en FODMAPs durante 2–3 semanas. La segunda dura 8 semanas, y en ese período se introducen de nuevo de forma gradual alimentos ricos en FODMAPs. La última fase consiste en personalizar la dieta según la tolerancia individual. En este artículo se detallan aquellos alimentos que contienen dichos compuestos y los posibles sustitutos. Además, en una serie de Anexos se incluyen registros dietéticos específicos, y material didáctico para facilitar el cumplimiento de la dieta. Pese a que la mayoría de estudios se han realizado en población adulta, se ha observado que en edad pediátrica también tiene efectos beneficiosos. No obstante, se requiere más investigación al respecto.
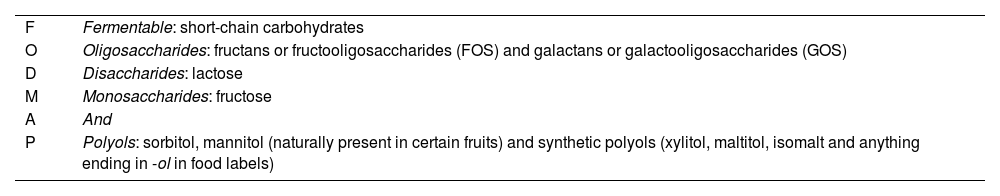
The acronym FODMAP (Table 1) stands for Fermentable, Oligosaccharides, Disaccharides, Monosaccharides and Polyols.
Description of the FODMAP acronym with examples.
| F | Fermentable: short-chain carbohydrates |
| O | Oligosaccharides: fructans or fructooligosaccharides (FOS) and galactans or galactooligosaccharides (GOS) |
| D | Disaccharides: lactose |
| M | Monosaccharides: fructose |
| A | And |
| P | Polyols: sorbitol, mannitol (naturally present in certain fruits) and synthetic polyols (xylitol, maltitol, isomalt and anything ending in -ol in food labels) |
The term FODMAP refers to short-chain carbohydrates that are absorbed poorly by the small intestine and pass directly to the colon, where they are fermented by the intestinal microbiota, giving rise to of gases such as methane, butyrate or propionate. The osmotic load that they produce increases the volume of water in the colonic lumen, which in turn causes symptoms such as abdominal distension and pain.1
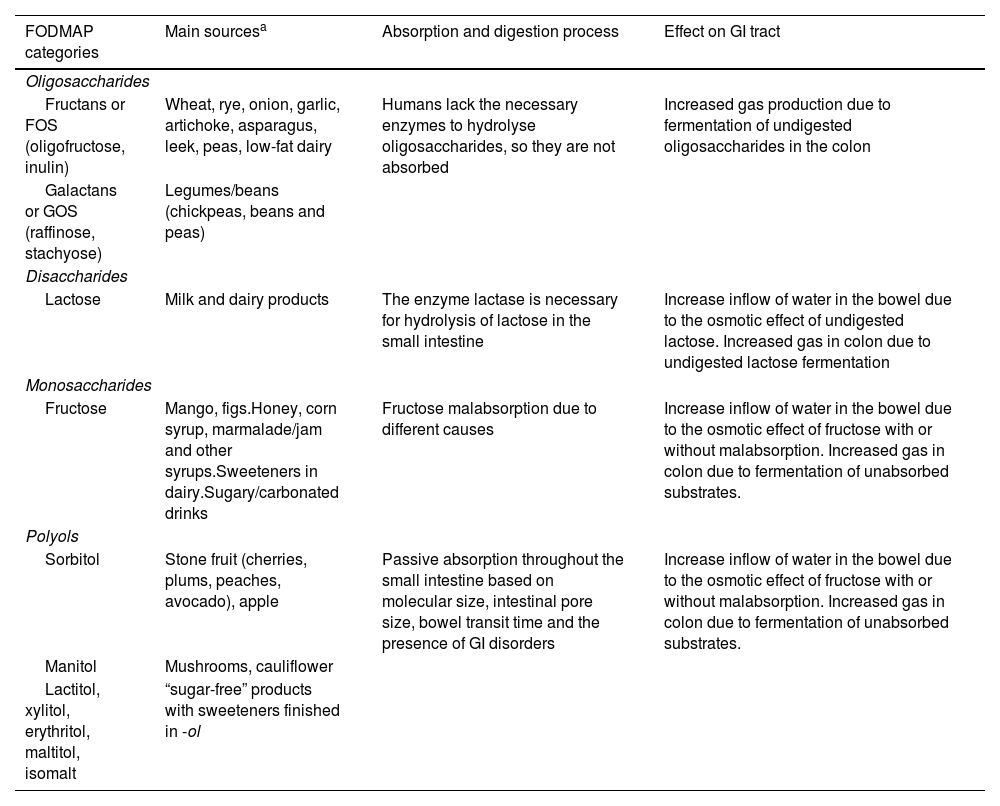
The foods that contain FODMAPs are numerous and ubiquitous in Western diets: dairy products, fruits, vegetables, legumes and beans, cereals, baked goods, sauces, candies, beverages, jams or frozen treats, among others. Table 2 presents the different FODMAP categories, examples of the main sources, the digestion and absorption process and their effects on the gastrointestinal (GI) tract.
FODMAPs and their effects on the gastrointestinal tract.
| FODMAP categories | Main sourcesa | Absorption and digestion process | Effect on GI tract |
|---|---|---|---|
| Oligosaccharides | |||
| Fructans or FOS (oligofructose, inulin) | Wheat, rye, onion, garlic, artichoke, asparagus, leek, peas, low-fat dairy | Humans lack the necessary enzymes to hydrolyse oligosaccharides, so they are not absorbed | Increased gas production due to fermentation of undigested oligosaccharides in the colon |
| Galactans or GOS (raffinose, stachyose) | Legumes/beans (chickpeas, beans and peas) | ||
| Disaccharides | |||
| Lactose | Milk and dairy products | The enzyme lactase is necessary for hydrolysis of lactose in the small intestine | Increase inflow of water in the bowel due to the osmotic effect of undigested lactose. Increased gas in colon due to undigested lactose fermentation |
| Monosaccharides | |||
| Fructose | Mango, figs.Honey, corn syrup, marmalade/jam and other syrups.Sweeteners in dairy.Sugary/carbonated drinks | Fructose malabsorption due to different causes | Increase inflow of water in the bowel due to the osmotic effect of fructose with or without malabsorption. Increased gas in colon due to fermentation of unabsorbed substrates. |
| Polyols | |||
| Sorbitol | Stone fruit (cherries, plums, peaches, avocado), apple | Passive absorption throughout the small intestine based on molecular size, intestinal pore size, bowel transit time and the presence of GI disorders | Increase inflow of water in the bowel due to the osmotic effect of fructose with or without malabsorption. Increased gas in colon due to fermentation of unabsorbed substrates. |
| Manitol | Mushrooms, cauliflower | ||
| Lactitol, xylitol, erythritol, maltitol, isomalt | “sugar-free” products with sweeteners finished in -ol | ||
On one hand, fructose is absorbed by the small intestine via the GLUT5 (high-affinity) or GLUT2 (low-affinity) transporter. Physiological fructose malabsorption occurs if the GLUT5 transporter is saturated, when the fructose intake exceeds 20 g in a single eating episode.2 Glucose aids the absorption of fructose via GLUT2, so foods containing fructose and glucose in a 1:1 ratio are better tolerated. However, when there is an excess of fructose relative to glucose (fructose excess = free fructose – free glucose), the former is not well absorbed, producing an increase in the water influx into the lumen. The fruits with fructose excess include pear, apple, mango and melon, and the vegetables, asparagus.2,3
When it comes to sorbitol and fructose, when consumed together, the former hinders the absorption of the latter, as they compete for GLUT5 uptake. The consequence of a fructose excess is the development of GI problems. Fruits with fructose excess combined with sorbitol exacerbate the GI symptoms (Table 2).
Lactose, once ingested, undergoes hydrolysation by lactase, (β-galactosidase).4,5 Following digestion (hydrolysis), the resulting glucose and galactose are absorbed through active transport. In humans, lactase activity peaks around age 2 years and decreases from age 3–4 years in a variable percentage of the population, depending on ethnicity (lactase activity is greater in the Mediterranean Basin compared to Asia, for example).5 Lactase deficiency tends to manifest from age 5–7 years, although in some populations the onset is delayed to adolescence, and entails the development of a constellation of symptoms such as flatulence, abdominal pain and distension and/or diarrhoea due to the arrival of lactose that has not been hydrolysed to the colon.
As regards fructooligosaccharides (FOS) and galactooligosaccharides (GOS), their malabsorption is due to humans not producing the enzymes required to hydrolyse them, so they are not absorbed.
On the other hand, polyols (see Table 2) are absorbed through passive diffusion along the length of the small intestine. The degree of absorption varies depending on the size of the molecule and of the intestinal pores, the time in the small bowel in relation to the absorption time window and the presence of GI disorders.4–6
The aim of this document is to establish a protocol for the use of the low-FODMAP diet in paediatric patients and to review the current evidence on the efficacy of this diet.
Material and methodsWe conducted a narrative review of sources obtained through the PubMed and Web of Science databases, Google Scholar and websites of agencies and other official institutions.
In the search conducted in PubMed, we applied selection criteria such as “human” and “child”, limiting the search to sources published in the past 5 years in English or Spanish language. In this search, we used the key words “fodmap” and “low fodmap diet” and the operator AND to combine either with the additional key word “children”.
On the other hand, in the search conducted in Web of Science, we applied selection criteria such as “human” and “children”, limiting the search to sources published in the past 5 years in English or Spanish language. we used the key words “fodmap” and “the low fodmap diet” and the operator AND to combine either with the additional key word “children”.
Evidence on the use of the low-FODMAP diet in paediatric patientsThe low-FODMAP diet is a therapeutic tool that can improve symptoms and nutrition in multiple GI disorders, such as irritable bowel syndrome (IBS),5,7 inflammatory bowel disease (IBD),8 small intestinal bacterial overgrowth (SIBO),9–11 non-coeliac gluten sensitivity (NCGS)8 or coeliac disease (CE).8 It has been found to be effective not only against IBS,7 but also in patients with fibromyalgia, sclerosis and endometriosis.12 Most studies in patients with IBS (and without CE) have been conducted in the adult population, demonstrating that dietary intake of gluten can cause symptoms. This suggests that individuals with IBS may either have NCGS or respond poorly to wheat fructans, which would explain why adherence to a low-FODMAP diet, which excludes them, can have beneficial effects.5
The evidence on the use of the low-FODMAP diet in the paediatric population is scarce.13 Notwithstanding, a significant improvement in symptoms related to gas production and abdominal pain and distention has been observed in paediatric patients on this diet.11,13 Constipation is the symptom that improves the least, which could be related to the low fibre intake in this dietary approach. In a study conducted by Baranguán Castro et al. in children aged 5–15 years (2019),14 most considered that the diet was easy to follow and there was a high proportion of adherence, associated with improved symptom control. Patients have also reported a decrease in abdominal pain associated with an improved quality of life.11,14
Final products of digestion that are not absorbed may produce gases, swelling, gastro-oesophageal reflux, heartburn, decreased appetite, abdominal distension /meteorism, borborygmus, colicky abdominal pain, epigastric pain and changes in bowel movements ranging from softer or more frequent stools to diarrhoea or even constipation.5 These symptoms have a deleterious impact on quality of life, so alleviating them is a priority.
For now, the efficacy of the diet in children with IBS has not been fully proven. However, there is evidence that suggests that implementation and adherence of the diet can improve its symptoms.5
In order to improve adherence to the low-FODMAP diet and its effectiveness, it is essential to perform a rigorous evaluation of abnormal dietary habits before and during the intervention. The reason is that there are complex psychosocial factors at play that are particularly significant in children and/or adolescents.8
The efficacy of the low-FODMAP diet is related to the baseline composition of the gut microbiota and its metabolic capacity. There are studies that suggest that the response ot the diet is better in children with IBS and a microbiota with a greater saccharolytic metabolic capacity. Therefore, this could be a predictive biomarker of the responsiveness to a low-FODMAP diet.8
Analyses of the gut microbiota after the implementation of the diet have revealed a decrease in microbial fermentation, evincing the efficacy in improving GI symptoms, especially abdominal pain, in children with IBS.5,11,15
The limitation of the duration of the elimination phase to 2 weeks in the paediatric population reduces the associated nutritional risks and facilitates adherence, which could contribute to the efficacy of the diet.13,14
As regards the total duration of the diet, previous studies suggest that issues with adherence emerge with a duration longer than 2 months, which reduces its efficacy.11,15 Determining the appropriate duration of this diet in children entails considering the minimum time required for efficacy, maximum time accepted for feasibility, and nutritional risks.8
Steps preceding initiation of the low-FODMAP dietTo identify diseases for which the low-FODMAP diet can be considered an appropriate dietary treatment, the Rome III and IV criteria can be applied.7 Furthermore, performance of certain tests before initiation of the diet is recommended to rule out certain parasitic infections or diseases that may require specific treatment.
- 1
Clinical evaluation/diagnostic tests. The evaluation is based on 2 screening tests:
- -
The hydrogen breath test using specific substrates that allow diagnosis of intolerance to certain carbohydrates (lactose, sucrose, sorbitol or fructose).5
- -
Oral tolerance tests. The most appropriate approach has to be discussed, given the possibility of false positive results due to the interaction with the oral microbiota, a different CO2 production rate or a lower CO2 distribution volume in younger children.6
- -
- 2
Physical examination, anthropometric measurement and testing for biomarkers of malnutrition (serum levels of calcium, iron, vitamin B12 and D, etc).
- 3
Psychological evaluation. The patient must be willing to collaborate and report truthful information.
- 4
Food and symptom diary (Appendix A-I in Supplementary material). The parents/guardians record for 3 days the foods consumed by the child following the usual diet, documenting any associated symptoms and the characteristics of the stools using the Brussels Infants and Toddlers Stool Scale (BITSS)16 in infants and toddlers or the Bristol stool chart in older children. Parents are provided with tables to help them estimate the amounts of food consumed. This document helps assess/determine the dietary preferences, unusual dietary habits and symptoms of the patient.5
The outcomes of the low-FODMAP diet improve when it is managed by a multidisciplinary team consisting, essentially, by a dietitian/nutritionist, GI doctor and mental health professional with specific training in this area. In many cases, symptoms improve within 2 weeks, so if there is no improvement after 3 or 4 weeks, the patient should not continue on the diet.
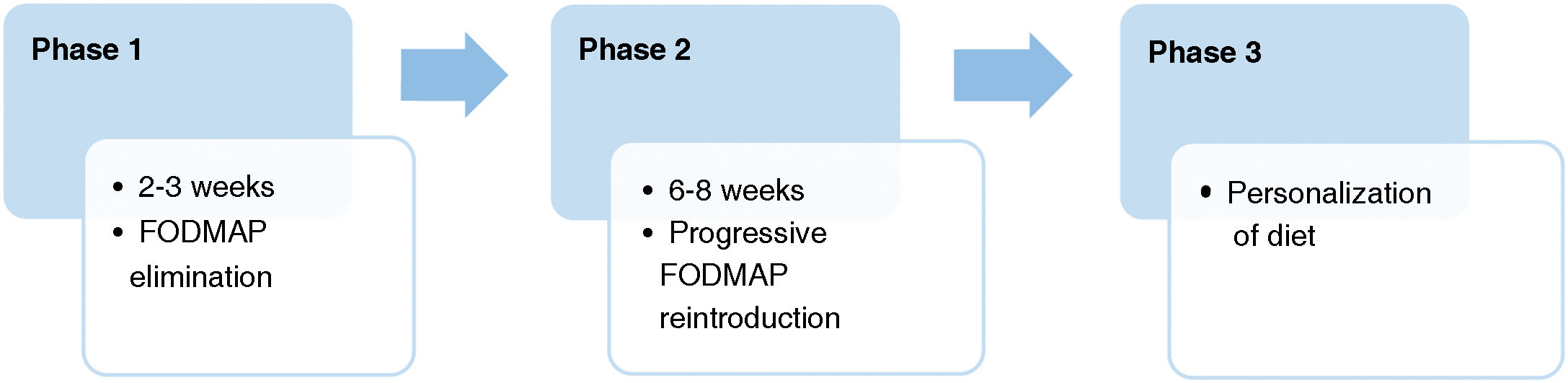
The diet is structured into 3 phases. In studies in paediatric patients,14 phase 1 lasts 2–3 weeks, phase 2–8 weeks and phase 3 has a variable duration. Fig. 1 summarises the phases of the diet, which has an approximate duration of 2–3 months in most patients.
First phase: elimination of FODMAP-containing foodsFoods with a high FODMAP content are eliminated for 2–3 weeks while monitoring changes in symptoms. If symptoms persist, both the proposed diet and the patient’s adherence to it need to be evaluated.
A duration of 2 weeks in the paediatric population may be as effective as 3 weeks in adults while decreasing the potential risks and facilitating adherence. In fact, some studies14 found that the majority of patients in the sample considered the diet easy to follow and a high adherence, which is associated with improved symptom control. At 2 weeks, patients exhibited an improvement in symptoms.13
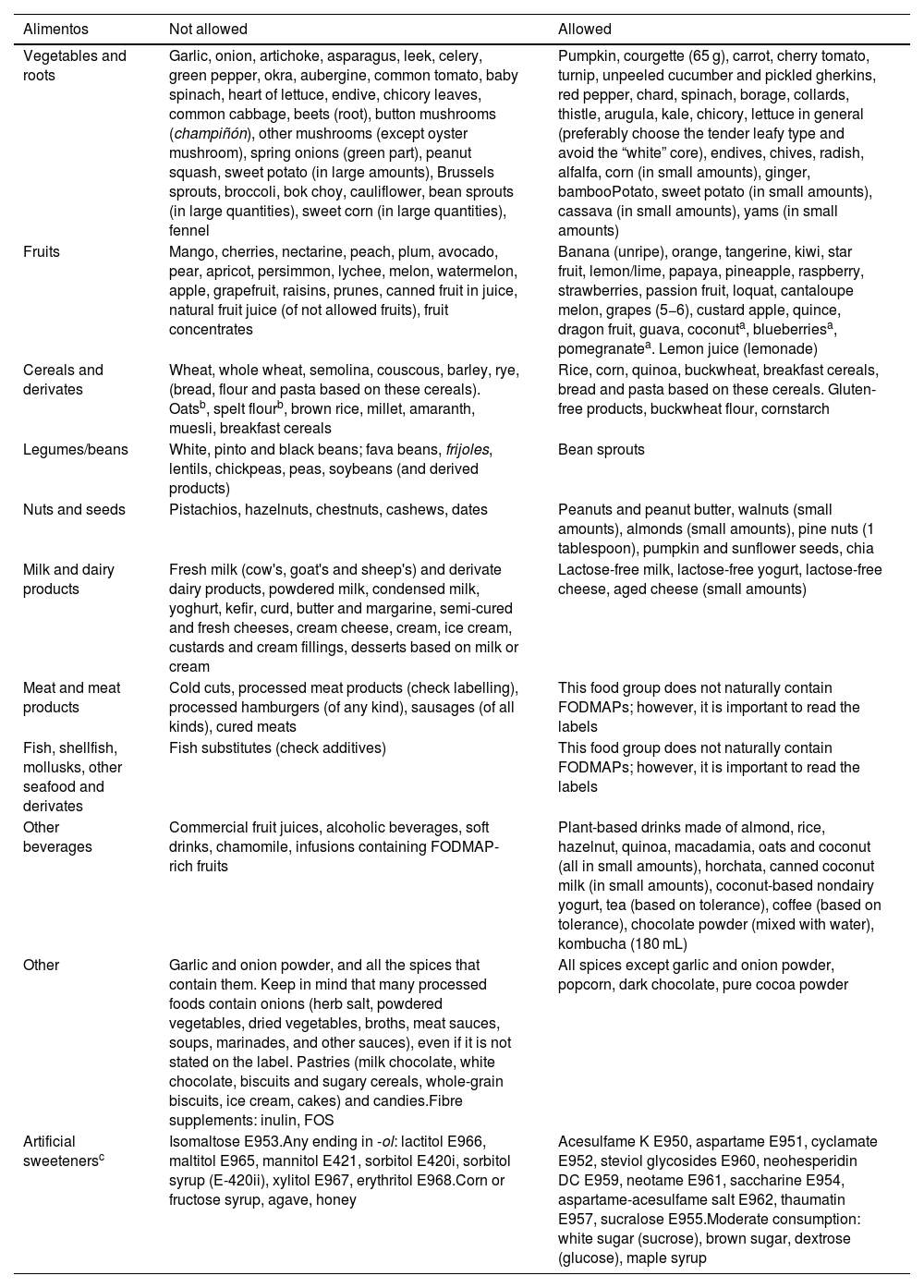
Tables 3 and 4 present lists of the foods that are allowed and not allowed and the potential nutrient deficiencies associated with the low-FODMAP diet.6,7,17,18 We ought to highlight that the allowed foods differ between studies, which complicates the implementation of the diet.
Lists of foods that are not recommended (“not allowed”) and recommended (“allowed”) in the low-FODMAP diet.
| Alimentos | Not allowed | Allowed |
|---|---|---|
| Vegetables and roots | Garlic, onion, artichoke, asparagus, leek, celery, green pepper, okra, aubergine, common tomato, baby spinach, heart of lettuce, endive, chicory leaves, common cabbage, beets (root), button mushrooms (champiñón), other mushrooms (except oyster mushroom), spring onions (green part), peanut squash, sweet potato (in large amounts), Brussels sprouts, broccoli, bok choy, cauliflower, bean sprouts (in large quantities), sweet corn (in large quantities), fennel | Pumpkin, courgette (65 g), carrot, cherry tomato, turnip, unpeeled cucumber and pickled gherkins, red pepper, chard, spinach, borage, collards, thistle, arugula, kale, chicory, lettuce in general (preferably choose the tender leafy type and avoid the “white” core), endives, chives, radish, alfalfa, corn (in small amounts), ginger, bambooPotato, sweet potato (in small amounts), cassava (in small amounts), yams (in small amounts) |
| Fruits | Mango, cherries, nectarine, peach, plum, avocado, pear, apricot, persimmon, lychee, melon, watermelon, apple, grapefruit, raisins, prunes, canned fruit in juice, natural fruit juice (of not allowed fruits), fruit concentrates | Banana (unripe), orange, tangerine, kiwi, star fruit, lemon/lime, papaya, pineapple, raspberry, strawberries, passion fruit, loquat, cantaloupe melon, grapes (5−6), custard apple, quince, dragon fruit, guava, coconuta, blueberriesa, pomegranatea. Lemon juice (lemonade) |
| Cereals and derivates | Wheat, whole wheat, semolina, couscous, barley, rye, (bread, flour and pasta based on these cereals). Oatsb, spelt flourb, brown rice, millet, amaranth, muesli, breakfast cereals | Rice, corn, quinoa, buckwheat, breakfast cereals, bread and pasta based on these cereals. Gluten-free products, buckwheat flour, cornstarch |
| Legumes/beans | White, pinto and black beans; fava beans, frijoles, lentils, chickpeas, peas, soybeans (and derived products) | Bean sprouts |
| Nuts and seeds | Pistachios, hazelnuts, chestnuts, cashews, dates | Peanuts and peanut butter, walnuts (small amounts), almonds (small amounts), pine nuts (1 tablespoon), pumpkin and sunflower seeds, chia |
| Milk and dairy products | Fresh milk (cow's, goat's and sheep's) and derivate dairy products, powdered milk, condensed milk, yoghurt, kefir, curd, butter and margarine, semi-cured and fresh cheeses, cream cheese, cream, ice cream, custards and cream fillings, desserts based on milk or cream | Lactose-free milk, lactose-free yogurt, lactose-free cheese, aged cheese (small amounts) |
| Meat and meat products | Cold cuts, processed meat products (check labelling), processed hamburgers (of any kind), sausages (of all kinds), cured meats | This food group does not naturally contain FODMAPs; however, it is important to read the labels |
| Fish, shellfish, mollusks, other seafood and derivates | Fish substitutes (check additives) | This food group does not naturally contain FODMAPs; however, it is important to read the labels |
| Other beverages | Commercial fruit juices, alcoholic beverages, soft drinks, chamomile, infusions containing FODMAP-rich fruits | Plant-based drinks made of almond, rice, hazelnut, quinoa, macadamia, oats and coconut (all in small amounts), horchata, canned coconut milk (in small amounts), coconut-based nondairy yogurt, tea (based on tolerance), coffee (based on tolerance), chocolate powder (mixed with water), kombucha (180 mL) |
| Other | Garlic and onion powder, and all the spices that contain them. Keep in mind that many processed foods contain onions (herb salt, powdered vegetables, dried vegetables, broths, meat sauces, soups, marinades, and other sauces), even if it is not stated on the label. Pastries (milk chocolate, white chocolate, biscuits and sugary cereals, whole-grain biscuits, ice cream, cakes) and candies.Fibre supplements: inulin, FOS | All spices except garlic and onion powder, popcorn, dark chocolate, pure cocoa powder |
| Artificial sweetenersc | Isomaltose E953.Any ending in -ol: lactitol E966, maltitol E965, mannitol E421, sorbitol E420i, sorbitol syrup (E-420ii), xylitol E967, erythritol E968.Corn or fructose syrup, agave, honey | Acesulfame K E950, aspartame E951, cyclamate E952, steviol glycosides E960, neohesperidin DC E959, neotame E961, saccharine E954, aspartame-acesulfame salt E962, thaumatin E957, sucralose E955.Moderate consumption: white sugar (sucrose), brown sugar, dextrose (glucose), maple syrup |
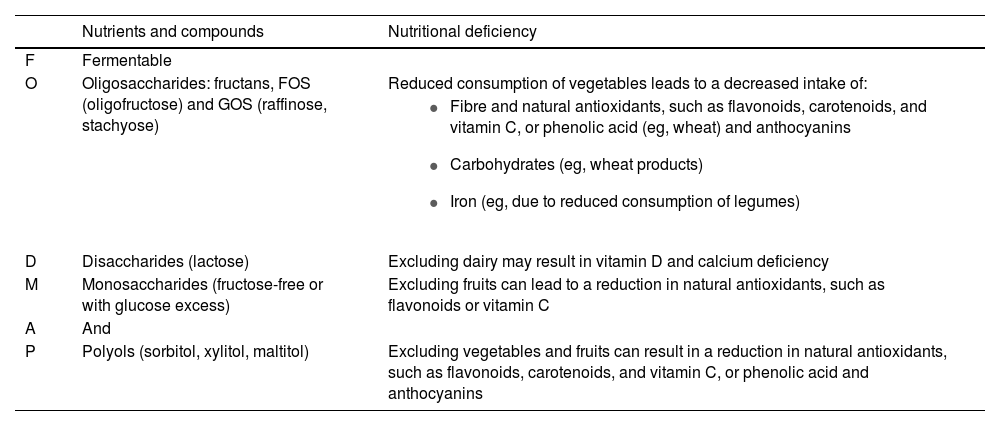
Possible nutrient deficits associated with the low-FODMAP diet.
| Nutrients and compounds | Nutritional deficiency | |
|---|---|---|
| F | Fermentable | |
| O | Oligosaccharides: fructans, FOS (oligofructose) and GOS (raffinose, stachyose) | Reduced consumption of vegetables leads to a decreased intake of: |
| ||
| D | Disaccharides (lactose) | Excluding dairy may result in vitamin D and calcium deficiency |
| M | Monosaccharides (fructose-free or with glucose excess) | Excluding fruits can lead to a reduction in natural antioxidants, such as flavonoids or vitamin C |
| A | And | |
| P | Polyols (sorbitol, xylitol, maltitol) | Excluding vegetables and fruits can result in a reduction in natural antioxidants, such as flavonoids, carotenoids, and vitamin C, or phenolic acid and anthocyanins |
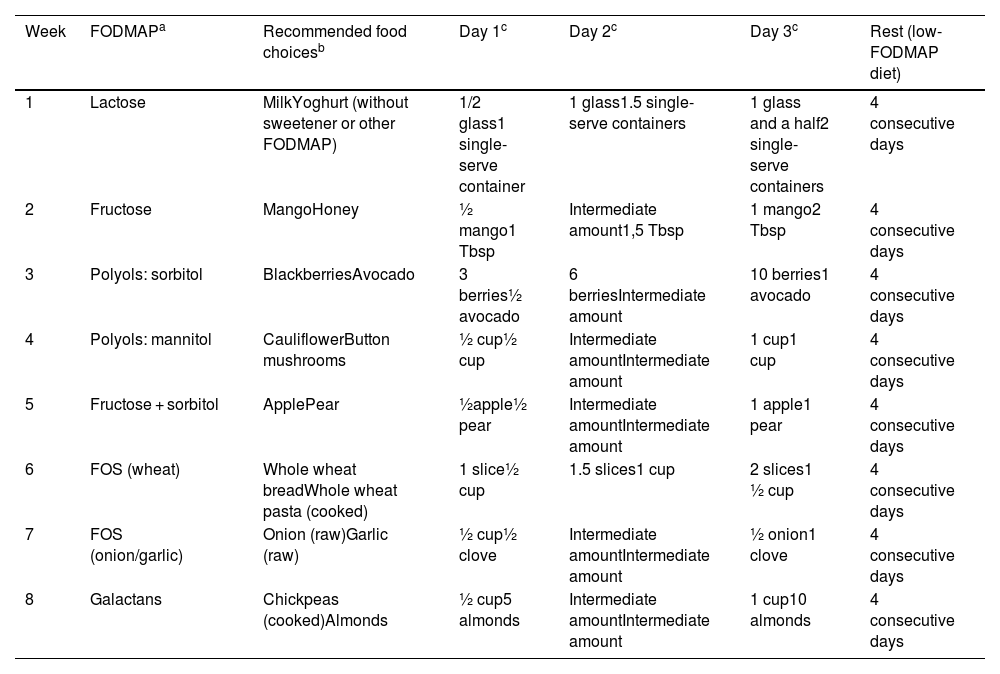
Before starting the second phase, the team considers whether the previous phase has succeeded in alleviating the symptoms; if it has, FODMAP-rich foods are reintroduced in a planned schedule to determine which cause symptoms and which do not. The order in which the different FODMAP groups are reintroduced in children is the same as in adults and is presented in Table 5.
Order of FODMAP reintroduction.
| Week | FODMAPa | Recommended food choicesb | Day 1c | Day 2c | Day 3c | Rest (low-FODMAP diet) |
|---|---|---|---|---|---|---|
| 1 | Lactose | MilkYoghurt (without sweetener or other FODMAP) | 1/2 glass1 single-serve container | 1 glass1.5 single-serve containers | 1 glass and a half2 single-serve containers | 4 consecutive days |
| 2 | Fructose | MangoHoney | ½ mango1 Tbsp | Intermediate amount1,5 Tbsp | 1 mango2 Tbsp | 4 consecutive days |
| 3 | Polyols: sorbitol | BlackberriesAvocado | 3 berries½ avocado | 6 berriesIntermediate amount | 10 berries1 avocado | 4 consecutive days |
| 4 | Polyols: mannitol | CauliflowerButton mushrooms | ½ cup½ cup | Intermediate amountIntermediate amount | 1 cup1 cup | 4 consecutive days |
| 5 | Fructose + sorbitol | ApplePear | ½apple½ pear | Intermediate amountIntermediate amount | 1 apple1 pear | 4 consecutive days |
| 6 | FOS (wheat) | Whole wheat breadWhole wheat pasta (cooked) | 1 slice½ cup | 1.5 slices1 cup | 2 slices1 ½ cup | 4 consecutive days |
| 7 | FOS (onion/garlic) | Onion (raw)Garlic (raw) | ½ cup½ clove | Intermediate amountIntermediate amount | ½ onion1 clove | 4 consecutive days |
| 8 | Galactans | Chickpeas (cooked)Almonds | ½ cup5 almonds | Intermediate amountIntermediate amount | 1 cup10 almonds | 4 consecutive days |
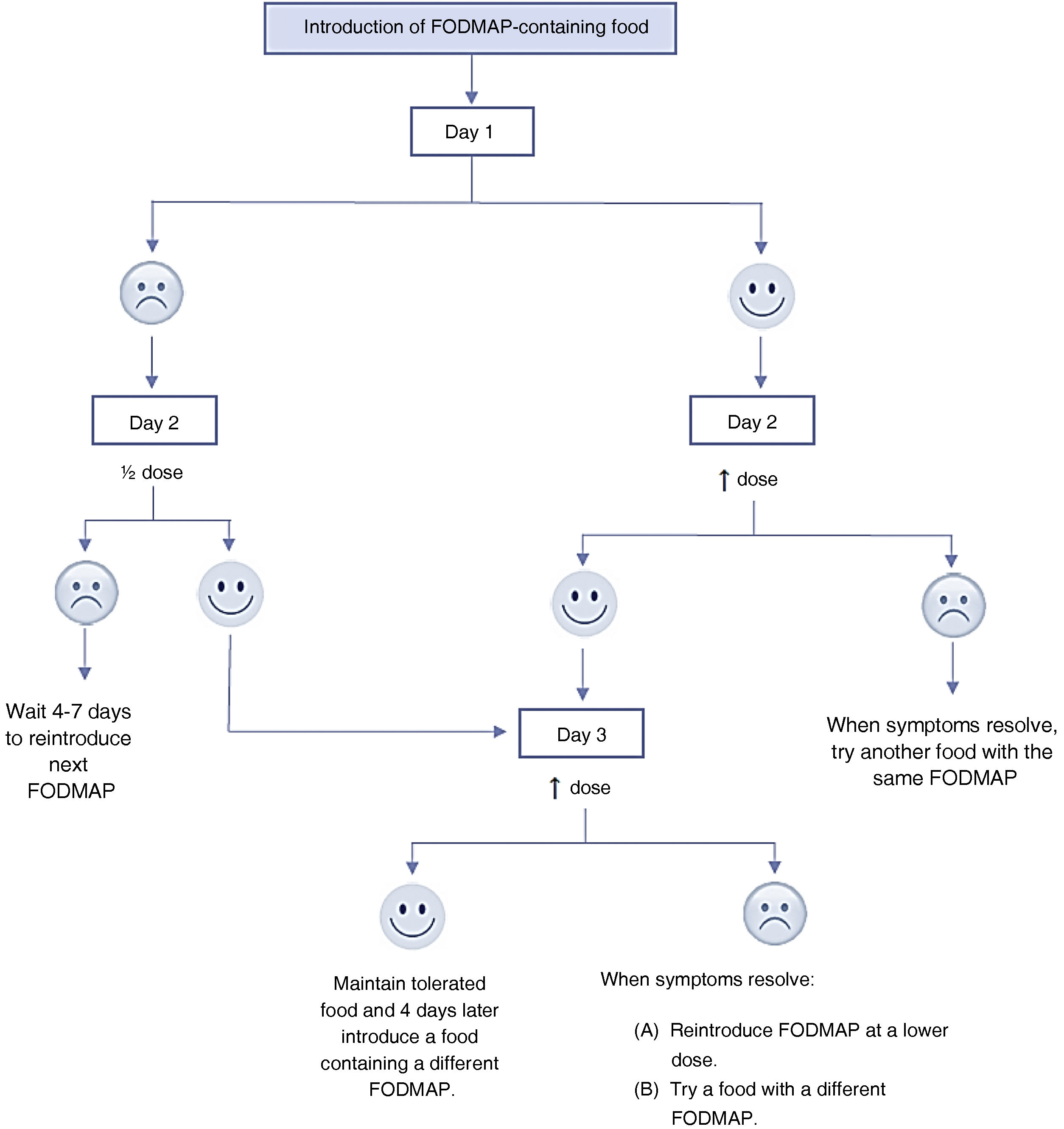
Each week, the indicated FODMAP is introduced for 3 days. For each FODMAP, the patient is given two choices of foods containing it and must pick only one of them, gradually increasing the amount consumed.14 During those days, a food and symptoms diary must be kept, recording the food item and the amount consumed per the prescription, in addition to any symptoms developed during or after its intake. This allows patients and/or the parents/guardians to identify specific FODMAP triggers and the dose at which they cause symptoms.8
After the 3 reintroduction days, the reintroduced foods are maintained in the diet at the tolerated dose for the remaining 4 days of the week while continuing with the previously prescribed low-FODMAP diet.
In patients that develop symptoms at any point in those 3 days, the dose should not be increased further or consumption of that food should be discontinued. After 4–7 days, once the symptoms resolve, another FODMAP can be introduced. Another possible approach is to continue reintroduction of the same food item the next day at half the dose or switching it to a different food containing the same FODMAP. If symptoms develop again after reducing the dose, it is likely that the patient will not be able to tolerate any foods in that FODMAP group well, so the patient should be advised to avoid them whenever possible.8,14Fig. 2 presents an algorithm for this process.
Thus, once the level of tolerance to different FODMAPs is established, their contents in the diet are adjusted to create a plan with the minimum possible restrictions to reduce the potential adverse effects on the gut microbiota, colonocyte metabolism and the patient’s long-term nutritional status.18 Anthropometric measurement during the follow-up is recommended, as patients tend to lose weight during this phase of treatment.4,5
Appendix A-II (Supplementary material) shows low-FODMAP plates to facilitate adherence during the reintroduction phase
We recommend starting with foods with the lowest FODMAP content. Appendix A-III (Supplementary material) details the amounts of FODMAP in some fruits and vegetables1 in increasing order. Using this information, patients can choose fruits that only have an excess of fructose, sorbitol, mannitol, fructose + sorbitol, etc. This is particularly helpful for the reintroduction of fructose, polyols and fructose + sorbitol.3
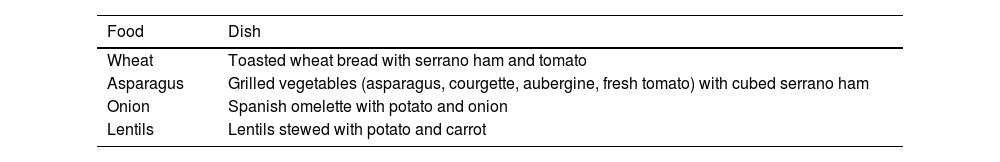
Table 6 presents a list of typical Spanish dishes that can be used to reintroduce foods after the elimination phase.12 However, the current evidence on the use of the low-FODMAP diet in Spain is scarce and hardly any local studies have estimated the feasibility and effectiveness of this strategy in reducing symptoms and improving health-related symptom reduction and quality of life in patients in Hispanic countries (Spain and Spanish-speaking countries in Latin America).12
Examples of typical Spanish dishes that can be reintroduced after the elimination phase of the low-FODMAP diet.
| Food | Dish |
|---|---|
| Wheat | Toasted wheat bread with serrano ham and tomato |
| Asparagus | Grilled vegetables (asparagus, courgette, aubergine, fresh tomato) with cubed serrano ham |
| Onion | Spanish omelette with potato and onion |
| Lentils | Lentils stewed with potato and carrot |
This phase concerns the use of a long-term dietary plan individualised according to the needs, tolerance, culture and preferences of the patient that is more varied and less restrictive and excludes only those FODMAPs that cause symptoms.
If there is no improvement in symptoms, other possible causes need to be considered.8 It is important that the patient remains in follow-up during this phase, although keeping a food and symptoms diary is no longer necessary.
Challenges of the low-FODMAP dietSince the low-FODMAP diet is quite restrictive, one of the associated risks is compromised nutrition; there is also evidence that patients may lose weight.5 Due to the decreased consumption of dairy, calcium levels and bone health should be monitored. However, calcium levels can be maintained through the consumption of lactose-free dairy products10 and/or consumption of other foods (such as sesame).18
The evidence regarding fibre intake is contradictory, as some studies show that it decreases due to the restriction of cereals, legumes and beans, while others have found no changes in fibre intake. As is the case of other restrictive diets, patients may end up having low or deficient levels of some micronutrients, such as vitamins (vitamin D or B vitamins) and natural antioxidants (flavonoids and carotenoids).18
RecommendationsThere is no question that the role played by a specialist, the dietitian/nutritionist, is crucial for the successful management of the patient, as it promotes the implementation of and adherence to a more balanced diet.4 The dietary plan must be personalised based on the individual tolerance of each patient aiming at achieving balanced nutrition. While treatment is ongoing, it is important to assess and promote adherence.5 Long-term adherence may be harder to achieve and can cause difficulties in the social life of the patient. Thus, it is important to provide guidance through nutritional and dietary education, with an emphasis on lifestyle and dietary habits and the identified needs of the patient, helping the patient learn and develop skills that will allow them to improve their own quality of life. Keeping daily records of dietary intake and symptoms can help identify foods that worsen clinical manifestations.7 Appendix A-IV (Supplementary material) provides dietary advice and recommendations for eating outside the home.
ConclusionThe narrative review allowed drawing the following conclusions:
- none-
The low-FODMAP diet is restrictive and must be supervised by a multidisciplinary team of experts in child nutrition.
- none-
If there is no clinical response within 3 weeks, the diet should not continue.
- none-
The tolerance to each food may vary between individuals and based on the clinical picture. In consequence, dietary recommendations must be personalised in terms of the type, amount and cooking/preparation of the foods.
- none-
The diet is structured into 3 phases; phase 1 lasts 2–3 weeks; phase 2 lasts 8 weeks and, lastly, phase 3 (the personalization phase) is of variable duration. The total duration of the diet is usually of 2–3 months.
- none-
Although several studies have shown that the low-FODMAP diet can improve symptoms in certain GI disorders, there is little evidence in the paediatric population. Therefore, high-quality studies need to be conducted before a consensus can be reached on the use of this diet in paediatric clinical practice.
- none-
Further studies with controls need to be conducted to assess the prolonged use (several months or longer) of the low-FODMAP diet and its impact on GI symptoms and physiology and gut microbiota composition and function. Such studies are essential to guarantee the long-term efficacy and safety of this dietary intervention.
This research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.