To compare the effectiveness and safety of polyethylene glycol with and without electrolytes (EL) over a 12 week period in treatment of chronic constipation in paediatrics.
Material and methodsThis was an observational, prospective, longitudinal, parallel group study, including 62 children with chronic constipation according to ROME III criteria and a history of faecal impaction. The children were divided into groups, one group of 30 received polyethylene glycol without EL (PEG) and 32 PEG with EL (PEG+EL) for at least 12 weeks. The main outcomes were the number of bowel movements at 6 and 12 weeks, and the presence of electrolyte disturbances at 6 weeks.
ResultsThe mean weekly stool frequencies were similar in both groups at 6 and 12 weeks, with 5.4 and 4.6 stools per week in the PEG+EL and PEG groups, respectively at 12 weeks. After 6 weeks of treatment, 83% (25 of 30) of the PEG group had at least one electrolyte disturbance compared with 56% (18 of 32) in the PEG+EL group (P=.02). Hyponatraemia was found in 15% (5 of 32) vs. 36% (11 of 30) of PEG+EL and PEG groups, respectively (P=.05). None of the laboratory abnormalities were clinically relevant.
ConclusionsPEG formulations with or without EL have a quite similar effectiveness, safety and acceptability. PEG without EL produced more electrolyte abnormalities, but none of them were symptomatic.
El objetivo del estudio fue comparar la efectividad y seguridad del polietilenglicol con y sin electrolitos (EL) en el estreñimiento funcional pediátrico a lo largo de 12 semanas.
Material y métodosEstudio observacional, prospectivo, longitudinal, de grupos paralelos, que incluye a 62 niños diagnosticados de estreñimiento funcional según los criterios de ROMA III con antecedente de impactación fecal. De ellos, 30 niños recibieron polietilenglicol sin EL (PEG) y 32 PEG con EL (PEG + EL) durante al menos 12 semanas. Los resultados principales fueron determinar el número de deposiciones por semana a las 6 y 12 semanas de tratamiento y la presencia de alteraciones hidroelectrolíticas a las 6 semanas.
ResultadosLa media de deposiciones por semana fue similar en ambos grupos a las 6 y a las 12 semanas, siendo en la semana 12 de 5,4 y 4,6 deposiciones por semana en los grupos PEG + EL y PEG respectivamente. Después de 6 semanas de tratamiento, el 83% (25 de 30) del grupo PEG tuvo al menos un parámetro alterado en la analítica, comparado con el 56% (18 de 32) en el grupo PEG + EL (p = 0,02). Se reportó una hiponatremia hasta en un 15% (5 de 32) y un 36% (11 de 30) del grupo PEG + EL y el grupo PEG (p = 0,05). Ninguna de las alteraciones analíticas fue clínicamente relevante.
ConclusionesLas formulaciones PEG con o sin EL tienen una efectividad, seguridad y aceptabilidad similar. PEG sin EL presentó un mayor número de alteraciones electrolíticas, pero ninguna fue sintomática.
Functional constipation in children is a common problem worldwide. Many affected individuals do not seek medical care, so it is difficult to determine its prevalence, but it has been estimated at between 0.7% and 29.6%.1 In Spain, reported prevalences in adults are as high as 14–20%.2,3 Constipation significantly impacts quality of life, causing physical as well as emotional distress.4 It is diagnosed by the Rome III criteria,5 which are very similar to those established by the PACCT group.6 The management of functional constipation includes several steps such as education, lifestyle changes, disimpaction and maintenance treatment.7 Some of the laxatives recommended for its treatment are magnesium hydroxide, lactulose, paraffin and polyethylene glycol (PEG). Compared to its predecessors, now in use for several decades, PEG has become the preferred choice of many practitioners.8 While the FDA has approved its use only in adults, its use in paediatrics has increased in several countries.9,10 Several studies have demonstrated its effectiveness and safety in the short term, and two recent systematic reviews11,12 concluded that PEG may be superior to lactulose and magnesium hydroxide. At present, two formulations of PEG are available in the market, one with and another without electrolytes, and despite their widespread use no studies have been conducted in children to compare their effectiveness and assess their long-term safety. Our study compared the effectiveness of PEG with electrolytes (PEG+EL) and without electrolytes (PEG) and their safety in relation to renal function and electrolyte values as biological markers of absorption over a period of twelve weeks.
Patients and methodsStudy designWe conducted an observational and prospective parallel study in two groups of patients that compared the safety and efficacy of a PEG laxative with electrolytes and another without for the treatment of chronic constipation.
The researchers obtained the consent of the patents before starting the period of observation.
Products under studyPEG without electrolytes (Casenlax® powder for oral solution, 4g and 10g packets).
Faecal impaction: 1.5–2g/kg/day in two doses until resolution for a maximum of six days (fixed dose).
Constipation: 0.4–1g/kg/day in two doses to a maximum of 20g/day. The duration of treatment was of at least 12 weeks.
PEG with electrolytes (Movicol®, powder for oral solution, 6.9 and 13.9g packets).
Faecal impaction: 1.5–2g/kg/day in two doses until resolution for a maximum of six days (fixed dose).
Constipation: 0.4–1g/kg/day in two doses to a maximum of 27.8g/day. The duration of treatment was of at least 12 weeks.
We included the patients referred to the Department of Paediatric Gastroenterology of the Hospital Sant Joan de Déu, between November 2011 and March 2013. The inclusion criteria were: age between 1 and 17 years, failure of educational and dietary interventions implemented for a minimum of one month, having had faecal impaction as part of the ongoing process, and meeting the Rome III criteria for the diagnosis of chronic functional constipation (presence of at least two of the following prior to diagnosis, for at least one month in children aged less than 4 years13 or two months in older children5: 2 defecations or fewer per week, at least one episode of faecal incontinence per week, history of retentive posturing [age>4 years], History of painful or hard bowel movements, presence of faecal masses in the rectum, and history of large diameter stools that may obstruct the toilet).
The exclusion criteria included patients that had used medications that can affect intestinal motility such as laxatives, probiotics or prebiotics in the four weeks preceding the first visit; patients with defecation problems of an organic aetiology, such as Hirschsprung disease, spina bifida (occult form), hypothyroidism, coeliac disease, metabolic or renal disorders, and patients with suspected gastrointestinal obstruction or stenosis.
All patients included in the study were assigned to one treatment or another in an alternating fashion in order to obtain a similar number of patients in each group. The administered dose was determined based on whether the patient presented with or without faecal impaction (as specified above). The possibility of changing the dose was considered if the patient did not respond to treatment after three days or developed any adverse effects, such as diarrhoea or abdominal pain.
To improve patient acceptance of both products, we allowed their suspension in fruit juice or milk.
We requested that parents keep a diary during the followup, documenting the frequency of depositions per week, their shape and their consistency according to the Bristol scale, which rates the consistency of faeces on a scale from 1 to 7.14 Appointments were scheduled at weeks 1, 4 and 12 from initiation of treatment, and subsequently every 8 weeks until treatment could be discontinued. Each visit included a full history taking and physical examination, including a rectal exam. Potential adverse effects of the treatment were also documented: abdominal pain, diarrhoea, flatulence, nausea and product acceptance. The need to increase or decrease the dose of the laxative based on the patient's clinical condition and bowel movements recorded in the journal was evaluated during each visit.
The primary outcome measures were the number of bowel movements per week recorded at weeks 6 and 12 of treatment, and the presence of electrolyte imbalances at week 6. Secondary outcomes included the time required to achieve disimpaction, change in the consistency of the stools, use of laxatives and tolerance (episodes of nausea or abdominal pain).
A blood sample was collected at the beginning of the study, six weeks of treatment and whenever the clinician deemed it appropriate during follow-up visits. All were fasting samples, and were collected in the laboratory of the hospital. The tests performed included complete blood count, electrolyte panel, serum osmolality, glucose, urea, creatinine, total serum protein and albumin. A first-void urine sample was also collected for electrolyte and osmolality testing. Table 1 presents the laboratory result ranges.
Biological parameters.
| Range | PEG+EL | PEG | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sodium, mmol/L (136–145) | ||||
| 5 | 15.6 | 11 | 36.6 | |
| >ANR | 0 | 0 | 0 | 0 |
| NR | 27 | 84.4 | 19 | 63.4 |
| Potassium, mmol/L (3.8–5.2) | ||||
| 1 | 3 | 2 | 6 | |
| >ANR | 0 | 0 | ||
| NR | 31 | 96 | 28 | 93 |
| Chlorine, mmol/L (97–110) | ||||
| 0 | 0 | |||
| >ANR | 0 | 0 | ||
| NR | 32 | 100 | 30 | 100 |
| Calcium, mmol/L (2.27–2.66) | ||||
| 3 | 4 | 13 | ||
| >ANR | 0 | 2 | 6 | |
| NR | 29 | 90 | 24 | 80 |
| Glucose (mg/dL) 70–109 | ||||
| 2 | 6 | 0 | ||
| >ANR | 0 | 0 | ||
| NR | 30 | 93 | 30 | 100 |
| Urea (mg/dL) 18–45 | ||||
| 0 | 0 | |||
| >ANR | 0 | 0 | ||
| NR | 32 | 100 | 30 | 100 |
| Creatinine (mg/dL)<0.64 | ||||
| 0 | 0 | |||
| >ANR | 2 | 6 | 0 | |
| NR | 30 | 93 | 28 | 100 |
| Serum osmolality (278–298) | ||||
| 0 | 1 | 3 | ||
| >ANR | 1 | 3 | 0 | |
| NR | 31 | 96 | 29 | 96 |
| Urine osmolality (513–1110) | ||||
| 2 | 9 | 8 | 18 | |
| >ANR | 2 | 9 | 0 | |
| NR | 19 | 82 | 20 | 71 |
| Urine sodium, mmol/L (34–214) | ||||
| 2 | 9 | 2 | 7 | |
| >ANR | 1 | 4 | 1 | 3 |
| NR | 20 | 86 | 25 | 89 |
| Urine potassium, mmol/L (27–93) | ||||
| 2 | 8 | 3 | 10 | |
| >ANR | 3 | 13 | 6 | 21 |
| NR | 18 | 78 | 19 | 67 |
| Chlorine, mmol/L (4–218) | ||||
| 2 | 9 | 0 | ||
| >ANR | 1 | 5 | 1 | 4 |
| NR | 18 | 85 | 23 | 95 |
BNR, below normal range; ANR, above the normal range; NR, normal range.
No changes were made to the usual diet of patients during the entire period under study.
Statistical analysisBased on previous studies,15,16 we decided that a difference of more than 30% between the treatments would be considered clinically relevant. Assuming an average frequency of seven bowel movements per week with a standard deviation of 3.5, we calculated that a sample of 60 (30 in each group) would be needed to require a 30% difference between the two groups in the number of bowel movements per week with a power of 80% (α=0.05).
We performed an intention-to-treat analysis of efficacy and biological and clinical safety that included all the data of enrolled patients that had received at least one dose of the laxatives under study and provided follow-up data for one or more of the analysed variables.
We performed comparisons between groups and within groups (before and after treatment) with the appropriate tests. We used the Kolmogorov–Smirnov test to ascertain the normality of the distributions. Quantitative parameters were compared by means of the Wilcoxon signed-rank test for paired samples and the Mann–Whitney U test for independent samples, and we described the data using the mean±standard deviation (SD) and the median. We summarised qualitative variables as frequencies and percentages, and compared them by means of the χ2 test or Fisher's exact test. The statistical analysis was performed with the software SPSS version 21. The level of statistical significance was 5%, and P-values of less than .05 were considered significant.
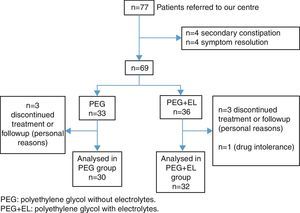
ResultsBetween November 2011 and March 2013 we recruited a total of 77 patients with constipation. Four of them were excluded due to having secondary constipation (three with coeliac disease and one with Hirschsprung disease) and another four because they improved with hygiene measures that were part of the routine management of functional constipation. After recruitment, patients were assigned to the two treatment groups. We lost another seven patients during followup, and one patient discontinued treatment due to intolerance to the drug. A total of 62 patients completed the 12 weeks of treatment, 30 in the PEG group and 32 in the PEG+EL group (Fig. 1). The average age was 5 years, and 53% of the patients were male. We found no relevant clinical or demographic differences between both treatment groups at the beginning of the study (Table 2).
Demographic characteristics of the patients.
| Variables | PEG+EL (N=32) | PEG (N=30) | P |
|---|---|---|---|
| Age in years, mean±SD | 62.3 (50.63) | 57.2 (41.34) | .75 |
| 1–3 years | 15 | 15 | |
| 3–5 years | 7 | 4 | |
| >5 years | 10 | 11 | |
| Male, n (%) | 18 (56) | 15 (50) | .62 |
| Height in cm, mean±SD | 106 (24) | 102 (22) | .5 |
| Weight in kg, mean±SD | 20 (11) | 18 (10) | .39 |
| Duration of constipation in months (mean) | 34 (28) | 33 (31) | .77 |
| Number of bowel movements/week | 1.66 | 1.63 | .69 |
| Stool consistency (Bristol stool scale) mean±SD | 1.53 (0.50) | 1.57 (0.56) | .87 |
| Painful defecation, n (%) | 29 (90) | 28(93) | .69 |
| Volitional stool retention, n (%) | 10 (31) | 17(56) | .04 |
| Faecal incontinence, n (%) | 7 (21) | 13 (43) | .07 |
| Rectal bleeding, n (%) | 13 (40) | 8 (26) | .24 |
| Faecal impaction, n (%) | 12 (37) | 9 (30) | .53 |
The number of bowel movements per week was similar in both groups at weeks 6 and 12. The mean (SD) number of bowel movements per week in the PEG+EL and the PEG groups were 6.0 (2.7) and 6.1 (2.5) at 6 weeks, and 5.4 (1.8) and 4.6 (2.2) at 12 weeks, respectively. The mean number of days elapsed until patients first passed soft stools was similar in both groups: 2.7 (1–10) days in the PEG group and 2 (1–7) days in the PEG+EL group, a difference that was not statistically significant (Table 3).
The proportion of patients with hard stools (Bristol ≤3) decreased considerably in the PEG group and to a lesser degree in the PEG+EL group, a difference that was not statistically significant. At 6 weeks, 21% (7 out of 32) had hard stools, compared to 6% (2 out of 30), respectively, but the differences between groups disappeared by 12 weeks, with hard stools found in 9% (9 out of 30) and 5% (5 out of 18) of patients, respectively (Table 4).
Faecal impaction at the time of enrolment was present in 37% of the PEG+EL group (12 out of 32) and 30% of the PEG group (9 out of 30). At 6 weeks of treatment, this symptom persisted in a higher percentage of patients in the PEG+EL group (21% [7/32]) compared to the PEG group (10% [3/30]), a difference that was not statistically significant. At 12 weeks, the difference had disappeared, with impaction found in only 6% (3 out of 31) and 9% (2 out of 30) of patients, respectively. The number of days after which patients first passed soft stools was very similar: it was 2 days on average in the PEG group, and 2.3 days in the PEG+EL group.
The mean doses at 6 weeks were 0.61g/kg/day (PEG) and 0.63g/kg/day (PEG+EL) (P=.8), and at 12 weeks, they were 0.56g/kg/day (PEG) and 0.61 (PEG+EL) (P=.49), with no statistically significant differences.
Biological toleranceAfter six weeks of treatment, 56% of patients in the PEG+EL group (18 out of 32) and 83% in the PEG group (25 out of 30) had at least one abnormal laboratory result, a difference that was statistically significant (P=.02). This difference persisted when we analysed patients with more than two abnormal parameters, found in 28% of patients in the PEG+EL group (9 out of 32) and in 50% of the PEG group (15 out of 30), although the difference was not statistically significant (Table 5).
None of the patients had abnormal results in the complete blood count, total serum protein, albumin or transaminase levels.
Of the laboratory values that we analysed (Table 1), serum sodium was the parameter that was most frequently disturbed with treatment, and we found hyponatraemia (sodium≤135mEq/L) in up to 15% of the PEG+EL group (5 out of 32) and 36% of the PEG group (11 out of 30), a difference that was statistically significant (P=.05). The second abnormal result corresponded to urine potassium, found in 21% (5 out of 23) and 32% (9 out of 28), respectively. Finally, changes in urine osmolality were found in 17% of the PEG+EL group (4 out of 23) and 28% of the PEG group (8 out of 28). We did not find statistically significant differences in the baseline parameters of both groups.
Clinical toleranceOnly three patients reported adverse effects that did not improve upon reducing the usual dose. The reported adverse effects were diarrhoea (one patient in the PEG group) and abdominal pain and flatulence (two patients in the PEG+EL group), and treatment was discontinued and switched in all of them. Only one patient in the PEG+EL reported that the product had a bad taste, leading to a change in treatment. None of the laboratory abnormalities were clinically relevant. All reported cases of hyponatraemia were mild, with a mean sodium level of 134mEq/L (133–135mEq/L).
DiscussionThis is the first study in the literature conducted in children that compares the effectiveness of PEG with and without electrolytes and the fluid and electrolyte imbalances that they may cause. We corroborated that PEG without electrolytes causes mild hyponatraemia in a greater number of patients compared to PEG+EL. These results are similar to those reported by Seinela et al. in a study of institutionalised senile adults, in whom only serum sodium levels were abnormal, although they did not assess for additional abnormalities in urine. Although all the electrolyte imbalances were asymptomatic, we must keep in mind that we conducted the study on patients with no chronic, renal, pancreatic or heart comorbidities, diseases in which these imbalances could be more relevant.
The studies that assess the safety of PEG without electrolytes in children, sometimes in comparison with other laxatives, report electrolyte imbalances in none or only a few isolated patients. However, all of these studies have particular characteristics that must be taken into account, most of them were not designed nor had a large enough sample to detect differences, such as two studies that assessed the safety of PEG at 3–4 days of treatment.17,18 Others did not report the normal ranges applied in their laboratories,19 or had a study design in which follow-up laboratory tests were optional, so that laboratory results were only available for seventeen patients at three months since initiation of treatment.20 A study by Dupont et al21 reported not having found electrolyte imbalances in any of the patients, but this result could have been influenced by their broader sodium normal range (132–145).
Several studies have demonstrated the safety of PEG+EL in adults,16 while there are only two studies in the paediatric age group22,23 that assess for laboratory abnormalities during treatment, neither of which found abnormalities, consisted with the findings of our study.
As for the efficacy of either laxative, so far the only study in the literature that has compared their use in children is the one conducted by Savino et al.24 They concluded that PEG without electrolytes was better tolerated and associated with a greater number of bowel movements per week than PEG+EL. However, they only compared both medications over a period of 4 weeks. In our study, we also observed that PEG without electrolytes was associated to a greater number of patients with soft stools at six weeks, but these differences had disappeared by 12 weeks. We also found a small difference in the resolution of faecal impaction, which was resolved more frequently in the PEG group at 6 weeks, that had also disappeared by 12 weeks.
One possible hypothesis is that it takes more time for PEG+EL to act, but since both laxatives have the same active ingredient, this could only be explained by the ingestion of lower doses of PEG+EL, probably due to its taste. Two studies in adults16,25 and one in children24 showed that PEG without electrolytes was better tolerated or more palatable. In the study by Savino et al., 26% of participants treated with PEG + EL reported that they found the taste bad or very bad compared to only 2% of those treated with PEG. This was also the case in a study on 100 adult volunteers, of who 84 preferred PEG and only 7 PEG+EL.25
The study by Savino et al24 did not specify whether the PEG+EL group was allowed to dissolve the drug in anything other than water. The patient information leaflet for Movicol® advises that it should only be dissolved in water. This may be the reason why its patient acceptance and palatability are inferior to those of Casenlax®.
In our study, we allowed both products to be dissolved in different beverages to improve patient acceptance, and it is possible that doses of PEG+EL are lower in the early weeks while patients find out which beverage improves its palatability, after which adherence to the prescribed dose would improve.
Furthermore, two studies in adults have also demonstrated that both products have a similar efficacy, as they found no differences between PEG with and without electrolytes.16,26
We observed few adverse effects in our study: they occurred in only 3 of the 62 analysed patients, which remarks the safety of polyethylene glycol compared to other medications, such as stimulant or saline laxatives and hyperosmolar agents such as lactulose or lactitol, which are associated with a higher incidence of transient diarrhoea (10%), flatulence (6%) and abdominal pain (2%).
In conclusion, both PEG formulations (with or without electrolytes) have a similar effectiveness, safety and patient acceptance. The electrolyte imbalances found more frequently in the group treated with PEG without electrolytes were very mild and caused no symptoms. It is possible that, compared to other studies, patient acceptance of PEG+EL in our study was greater because it could be dissolved in different beverages.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Llerena E, Varea Calderón V, Pujol Muncunill G, Hernandez Hernandez K, Sosa Giraldo FJ, Suarez Fuentes T, et al. Comparación sobre la efectividad y seguridad del polietilenglicol con y sin electrolitos. An Pediatr (Barc). 2016;85:34–40.