Describe the epidemiological and clinical pattern of Bordetella pertussis infection (whooping cough) among hospitalised infants less than one year-old in a paediatric hospital in Gran Canaria.
Patients and methodsA retrospective review of the patient hospital records was performed, and recording only those with a microbiological diagnosis of pertussis infection detected using polymerase chain reaction, from January 2008 to December 2016.
ResultsA total of 110 patients were identified, of which 105 (95.4%) were less than 6 months old, and 59.1% were males. The annual incidence of hospital admissions was estimated between 13.7 and 425.0 cases per 100000 infants <12 months old, with 2 peaks in 2011 and 2015. Household members were the main potential sources of infection. Main clinical features were pertussis cough associated with signs of catarrh, cyanosis, and lymphocytosis. Complications occurred in 15.4% of the patients (mainly pneumonia), but the outcome was favourable in all the cases. A lower age and non-vaccination were associated with an increased risk of developing complications (p<.05). Viral co-infection occurred in 31.6% of infants diagnosed with pertussis.
ConclusionsThe incidence of pertussis has increased in the last years in Gran Canaria, with a lower development of complications and mortality rates compared with the previous period. Lower age and non-vaccination status are considered risk factors for developing complications. Vaccination in pregnant women will probably lead to a decline in the incidence in the future, especially in infants younger than 6 months.
Describir el patrón epidemiológico y clínico de la infección por Bordetella pertussis (tosferina) en niños menores de un año hospitalizados en un hospital pediátrico de Gran Canaria.
Pacientes y métodosSe revisaron retrospectivamente las historias clínicas de los pacientes con diagnóstico microbiológico de infección por B. pertussis mediante reacción en cadena de la polimerasa, de enero de 2008 a diciembre de 2016.
ResultadosSe identificaron 110 pacientes, de los cuales 105 (95,4%) fueron menores de 6 meses y el 59,1% eran varones. La incidencia anual de hospitalización se estimó entre 13,7 y 425,0 casos por cada 100.000 lactantes menores de 12 meses, con 2 picos en 2011 y 2015. Los familiares cercanos fueron las principales fuentes de contagio potenciales. Las principales manifestaciones fueron la tos pertusoide asociada con signos catarrales, cianosis y linfocitosis. El 15,4% de los pacientes presentaron complicaciones (principalmente neumonía), pero la evolución fue favorable en todos los casos. La menor edad y la no vacunación se asociaron con un mayor riesgo de desarrollar complicaciones (p<0,05). La coinfección viral ocurrió en el 31,6% de los pacientes diagnosticados de infección por B. pertussis.
ConclusionesLa incidencia de infección por B. pertussis ha aumentado en los últimos años en nuestra área, con un menor desarrollo de complicaciones y con tasas de mortalidad inferiores al período anterior. La menor edad y la no vacunación previa se consideran factores de riesgo para el desarrollo de complicaciones. La vacunación en mujeres embarazadas probablemente disminuirá la incidencia en el futuro, sobre todo en niños menores de 6 meses.
Pertussis is a vaccine-preventable infectious disease caused by Bordetella pertussis. In recent years, there has been evidence of an increased incidence in many countries with a high vaccination coverage.1,2 Variations in published incidence rates may be due to differences in case reporting systems and access to diagnostic tests,2–4 but the highest rates are always reported in infants aged less than 1 year, who also experience the most complications and highest mortality. The Canary Islands is one of the autonomous communities in Spain with the highest rates of infection.5 In the 2003–2007 period, we performed a study where we found a high incidence of complications and mortality in this age group.6 For this reason, since 2015, vaccination of pregnant women was introduced in our autonomous community. In order to determine the subsequent trends of infection, we have conducted a clinical-epidemiological study of all the cases diagnosed in infants aged less than 1 year that visited the emergency department of the only public paediatric hospital in the island of Gran Canaria between 2008 and 2016.
Patients and methodsPatientsThe study included the 1,040 patients aged less than 1 year that visited the emergency department of the Hospital Materno Infantil de Gran Canaria (which serves an average of 6,770 infants aged less than 1 year) with a clinical presentation that required ruling out B. pertussis between January 2008 and December 2016.
MethodsAll patients underwent testing for detection of B. pertussis by means of polymerase chain reaction (PCR) (LightMix Kit Bordetella pertussis/parapertussis or Diagenode Bordetella pertussis/parapertussis) in samples of nasopharyngeal exudate obtained with a flexible swab. Viral detection tests were also performed 581patients (55.9%) in nasopharyngeal aspirate samples.
We performed a retrospective review of the health records of patients who tested positive for B. pertussis, collecting data on demographic variables, vaccination history, probably source of infection (contacts with clinical features compatible with pertussis), symptoms, laboratory data and treatment.
In the statistical analysis, we used the Mann–Whitney U test to compare quantitative variables and the chi square test to compare proportions. We defined statistical significance as a p-value of less than .05. The study was approved by the local bioethics and research committee.
ResultsEpidemiologyInfection by B. pertussis was detected in 110 patients (10.6%). A different pathogen was identified in 269 patients (25.7%): respiratory syncytial virus in 175, rhinovirus in 33, parainfluenzavirus 3 in 32, metapneumovirus in 7, cytomegalovirus in 5, influenza A in 5, influenza B in 4, enterovirus in 3, parainfluenzavirus 1 in 2, parainfluenzavirus 2 in 2, and coxsackievirus B in 1.
Of the 110 infants with infection by B. pertussis, 65 were male (59.1%). The mean age was 83.3 days (range, 14–287 days), and 105 (95.4%) were aged less than 6 months. Eleven had been born preterm (10.0%).
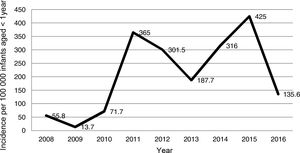
Fig. 1 shows the annual incidence of pertussis cases per 100000 infants aged less than 1 year that sought care in the hospital. The annual incidence ranged between 13.7 cases in 2009 and 425.0 cases in 2015, with 2 peaks in years 2011 and 2015. Since 2010, the year with the lowest incidence was 2016. When it came to the seasonal distribution, 33.6% of cases occurred in summer, 29.1% in spring, 19.1% in winter and 18.2% in autumn.
As for the vaccination status of the patients, 56 infants (50.9%) had not started to receive routine vaccinations, 47 (42.7%) had received 1 dose, 5 (4.5%) 2 doses and 2 (1.8%) 3 doses. Only 1 child was incorrectly vaccinated for age.
A probable source of infection was documented in 45 patients (40.9%), corresponding to household members in 44 cases (97.8%) and nosocomial infection in 1.
Seventeen infants with pertussis were born after the introduction of vaccination of pregnant women; in 5 of these infants (29.4%) the mother had received the vaccine in the third trimester of pregnancy (one at 28 weeks, one at 31 weeks, timing not documented in the remaining 3). The mean age of these 5 infants was 2.8 months (range, 0–5 months).
Clinical manifestationsTable 1 presents the clinical manifestations and abnormal laboratory findings in 110 patients. The characteristic clinical presentation consisted of whooping cough accompanied by cold symptoms, cyanosis and lymphocytosis. The only difference in clinical characteristics between older infants and infants aged less than 3 months was the development of pneumonia, which only occurred in those aged less than 3 months (p<.01). The mean time elapsed from onset of symptoms to the emergency department visit was 9.0±5.9 days (range, 1–28 days). A total of 107 patients were admitted to hospital (97.3%) for a mean length of stay of 10.7±7.3 days (range, 0–45 days).
Clinical manifestations and laboratory findings in the 110 patients with infection by B. pertussis.
| Age ≤3 months (%) n=65 | Age >3 months (%) n=45 | Total (%) n=110 | |
|---|---|---|---|
| Clinical manifestations | |||
| Cough | 65 (100) | 44 (97.8) | 109 (99.1) |
| Whooping cough | 56 (86.2) | 32 (71.1) | 88 (80.0) |
| Cold symptomsa | 43 (66.2) | 30 (66.7) | 73 (66.4) |
| Cyanosis | 35 (53.8) | 22 (48.9) | 57 (51.8) |
| Posttussive vomiting | 20 (30.8) | 12 (26.7) | 32 (29.1) |
| Fever | 8 (12.3) | 8 (17.8) | 16 (14.5) |
| Apnoea | 7 (10.8) | 2 (4.4) | 9 (8.2) |
| Pneumonia | 10 (15.4) | 0 (0) | 10 (9.1) |
| Tachypnoea | 3 (4.6) | 0 (0) | 3 (2.7) |
| Polypnoea | 2 (3.1) | 0 (0) | 2 (1.8) |
| Laboratory findingsb | n=64 | n=43 | n=107 |
| Lymphocytosis: absolute (>10000/mm3) or relative | 58 (90.6) | 41 (95.3) | 99 (92.5) |
| Leucocytosis (>17500/mm3) | 30 (46.9) | 15 (34.9) | 45 (42.1) |
| Thrombocytosis (>450000/mm3) | 43 (67.2) | 24 (55.8) | 67 (62.6) |
Complications developed in 17 patients (15.4%): 10 cases of pneumonia and 7 of apnoea. Table 2 presents the characteristics of the infants that developed complications: 82.3% had not started vaccination, and the rest had only received 1 dose. Nine infants with pneumonia were admitted to the intensive care unit (ICU). One girl aged 42 days (case 1) that was admitted to the ICU had originally presented with a leucocyte count of 27200cells/μL, but at 13 days from onset had developed hyperleukocytosis (103000 leucocytes/μL), so she underwent a double volume exchange transfusion. Table 3 shows the differences between infants that developed complications and infants that did not. Their comparison reveals that infants who developed complications were younger and that a higher proportion of patients in this group had not initiated routine vaccinations.
Characteristics of patients that developed complications.
| Case | Age (days)/sex | Vaccination | Leucocytes per×1000/mm3a | Coinfection | Complications | ICU admission | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 42/F | No | 103.0 | – | Pneumonia, hyperleukocytosis | Yes | AZM 5 days, MV, sedation/muscle relaxation, exchange transfusion, inhaled adrenaline, dexamethasone iv |
| 2 | 17/M | No | 18.1 | – | Pneumonia | Yes | AZM 5 days, MV, sedation/muscle relaxation, PN |
| 3 | 24/M | No | 32.0 | – | Pneumonia, ischaemic necrosis of RLE requiring amputation | Yes | AZM 5 days, MV, sedation/muscle relaxation, PN |
| 4 | 33/M | No | 16.7 | – | Pneumonia | Yes | AZM 5 days, MV, sedation/muscle relaxation, PN |
| 5 | 53/F | No | 46.3 | – | Pneumonia | Yes | MV, sedation/muscle relaxation |
| 6 | 54/M | No | 37.5 | – | Pneumonia | Yes | AZM 5 days, MV, sedation/muscle relaxation, PN |
| 7 | 65/M | 1 dose | 54.7 | – | Pneumonia | Yes | AZM 5 days, MV, sedation/muscle relaxation, PN |
| 8 | 46/F | No | 50.0 | RSV | Pneumonia, convulsive seizures | Yes | AZM 5 days, clonazepam |
| 9 | 47/F | No | 67.1 | – | Pneumonia | Yes | AZM 10 days, oxygen, PN, enoxaparin, Inhaled adrenaline |
| 10 | 72/F | No | No data | – | Pneumonia | No | AZM 5 days |
| 11 | 27/M | No | 7.3 | Coxsackievirus B | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 12 | 28/F | No | 10.3 | – | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 13 | 41/M | No | 10.2 | Rhinovirus | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 14 | 42/F | No | 32.7 | – | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 15 | 69/M | No | 11.5 | – | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 16 | 94/F | 1 dose | 21.0 | – | Apnoea secondary to coughing fits | No | AZM 5 days, upright position and oxygen during fits |
| 17 | 140/M | 1 dose | 10.1 | – | Apnoea (1 episode before admission) | No | AZM 5 days, upright position and oxygen during fits |
AZM, azithromycin; F, female; IV, intravenous; M, male; MV, mechanical ventilation; PN, parenteral nutrition; RLE, right lower extremity; RSV, respiratory syncytial virus.
Risk factors in patients that did and did not develop complications.
| Risk factor | Infants with complications (n=17) | Infants without complications (n=93) | p |
|---|---|---|---|
| Age in days, mean±SD | 52.6±29.9 | 88±53.7 | .003 |
| Preterm birth, n (%) | 2 (11.8) | 14 (15.1) | NS |
| Vaccination not initiated, n (%) | 14 (82.3) | 42 (45.2) | .005 |
| Coinfection, n (%) | 3 (17.6) | 15 (16.1) | NS |
| Leucocytes/μL | 24125±14097.0 | 16823.3±8701.4 | NS |
NS, not significant.
All patients received treatment from the time of admission with azithromycin (10mg/kg/day) for 5 days (except 1 patient treated for 10 days), with favourable outcomes.
Coinfection with other pathogensFifty-seven patients (51.8%) with infection B. pertussis underwent viral detection tests, which revealed in 18 (31.6%): by rhinovirus in 9, parainfluenzavirus 3 in 3, respiratory syncytial virus in 2, adenovirus in 1, influenza C in 1, cytomegalovirus in 1 and coxsackievirus B in 1.
DiscussionDespite the high vaccination coverage in the paediatric population, pertussis continues to be a significant health problem that recently led to the introduction of vaccination of pregnant women in the autonomous community of the Canary Islands with the intent to protect infants, the most vulnerable subset.7–9 Pertussis is a disease that causes epidemics on a cyclical pattern, although since 2010 in Spain there has been a sustained epidemic,5 which was reflected in our study. This phenomenon coincides with the re-emergence of pertussis observed both in the United States and in other European countries due to reasons that are yet unknown; some of the reasons that have been proposed are loss of immunity over time, antigenic drift in circulating strains relative to those used to make the vaccine and lack of vaccination in certain subsets of the population.9–13 In our region, between 2003 and 2010, the incidence in infants aged less than 1 year who required hospital admission only exceeded 100 cases in years 2003 (103 cases) and 2006 (204.5 cases).6 Since 2010, the annual incidence of cases with hospital admission exceeded 130 per 100000 inhabitants, with peaks of 365.0 and 425.0 cases in 2011 and 2015, respectively. However, in 2016 there was a decrease in incidence that coincided with the introduction of vaccination of pregnant women. This measure should be analysed in the long term to determine whether this trend is sustained, as it could also have been due to the data being collected in an interepidemic period.
Comparing incidence data between countries poses significant difficulties due to differences in the case definition, the diagnostic techniques used and reporting systems.2,14 According to data from the European Centre for Disease Prevention and Control, in 2014 the highest reported age-specific rate corresponded to infants aged less than 1 year, with an incidence of 51.6 cases per 100000 inhabitants in Europe overall. Spain, Lithuania, the Netherlands, Slovenia and Sweden were the countries with the highest incidence rates, with more than 100 cases per 100000 inhabitants in this age group.15 According to several studies, pertussis may be underdiagnosed, so its actual incidence could be higher. Basing the case definition on clinical variables could contribute to underdiagnosis, as such a definition is limited because signs and symptoms vary with age and vaccination status and also because physicians still maintain a low level of suspicion, especially in adolescents and adults. Recently, a research group proposed a diagnostic algorithm based on clinical signs and symptoms by age group.16 According to this algorithm, pertussis should be suspected in infants aged less than 3 months if they present with coughing and coryza, especially in cases where the cough increases in frequency and severity, independently of its duration or whether or not it is paroxysmal, or infants presenting with leucocytosis and lymphocytosis. In our study, most patients presented with cough, accompanied by cold symptoms in 2/3 of cases, and most also had lymphocytosis. We ought to note that pneumonia and apnoea developed mainly in infants aged less than 3 months, as described in previous studies,17 but we found no other differences in other signs and symptoms between the 2 age groups.
The population aged less than 1 year is at higher risk of complications. We observed complications in 15.4% of patients, mostly pneumonia. Younger age and unvaccinated status were the most significant factors associated to the development of complications, although none of the patients died. Compared to the preceding period, we found a lower rate of complications (23.4% in the 2003–2007 period) and a lower mortality (6.5% in the 2003–2007 period). Other studies have found a higher rate of complications in this age group.17 Several studies have analysed different variables and have found a higher risk of death or more severe disease in infants aged less than 4 months with low birth weight, hyperleukocytosis (≥100000 leukocytes/μL), who develop pulmonary hypertension or seizures or with unvaccinated status, among others,18–20 and a high degree of suspicion in these patients would probably improve clinical outcomes.
Viruses were detected in a high proportion of patients in whom B. pertussis infection was ruled out. The virus detected most frequently was respiratory syncytial virus, which demonstrates the overlap of signs and symptoms in this subset of the population, a fact that has been described by other authors.21,22 Furthermore, a viral coinfection was detected in a small proportion of the sample (most commonly by rhinoviruses), which was consistent with previous studies in which rhinoviruses and coronaviruses were most frequently detected in cases of coinfection.22
In conclusion, in recent years there has been an increase in the incidence of pertussis in infants aged less than 1 year in our region associated with decreases in the incidence of complications and in mortality compared to the previous period. Younger age and unvaccinated status were the main risk factors associated with the development of complications. The incidence of pertussis and its complications would probably decrease with vaccination of pregnant women, so it is important that long-term followup studies be conducted to assess the effectiveness of this preventive measure.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Iglesias L, Casabella Pernas A, Hernández Febles M, Colino Gil E, Eisman Maraver A, Pena López MJ. Estudio clínico-epidemiológico de la infección por Bordetella pertussis en la isla de Gran Canaria en el período 2008-2016. An Pediatr (Barc). 2018;89:170–175.
Previous presentation: This study was presented at the XXI National Congress of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (communication No. 300).