Rates of childhood cancer survival in developed countries have risen to over 80–85 %. In consequence, the population of childhood cancer survivors (CCS) has grown considerably. Nevertheless, CCS present a high morbidity and mortality due to cancer or its treatment, with an increased risk of premature mortality, second primary tumors and late side effects, both physical and psychosocial, all of which decrease the quality of life. Long-term follow-up (LTFU) of CCS is recommended to prevent, detect and treat those health problems. Despite the advances achieved, the management of CCS is still not optimal. Among the areas for improvement discussed in this manuscript are: (1) Quantifying the real burden of morbimortality, by implementing new frequency measures (mean cumulative count and cumulative burden), to obtain more accurate assessments, and using simulation models, to determine individual risks; (2) Assessing the impact of risk factors for late side effects, related to the patient, tumor type, treatments, lifestyle, comorbidities, genetics and ageing; (3) Considering the impact of the international harmonisation of long-term follow-up guidelines, to generate homogeneous, evidence-based recommendations and an individualized LTFU and, (4) Challenges to LTFU implementation, considering models of care adapted to patient risk and needs, with special attention to the transition to adult-care follow-up. Finally, we comment on the situation of CCS in Spain and consider future prospects for improving the health and quality of life of this population.
La supervivencia del cáncer infantil supera en la actualidad el 80–85 % en países desarrollados. La población superviviente ha crecido considerablemente, presentando una elevada morbimortalidad secundaria al cáncer o su tratamiento, representada por un riesgo incrementado de mortalidad prematura, segundos tumores y efectos tardíos (ET), en la esfera física y psicosocial, afectando la calidad de vida. El seguimiento a largo plazo y de por vida en supervivientes de cáncer infantil (SCI), está recomendado para prevenir, detectar y tratar sus problemas de salud. A pesar de los avances producidos, el manejo de los SCI no es adecuado. En este artículo se plantean los retos pendientes como: (1) Cuantificar la carga real de morbimortalidad, proponiendo nuevas medidas de frecuencia (recuento acumulado medio y carga acumulada), que ofrecen resultados más exactos de la multimorbilidad que presentan, y la utilización de modelos de simulación, para conocer el riesgo individual de eventos; (2) Impacto de factores que determinan el riesgo de presentar ET relacionados con el paciente, tumor, tratamientos, estilo de vida, comorbilidades, genética y envejecimiento; (3) Importancia de la armonización de las guías de seguimiento a largo plazo a nivel mundial, para ofrecer recomendaciones homogéneas, basadas en la evidencia y un plan individualizado de seguimiento y (4) Problemas en la implementación del seguimiento, con diferentes modelos de atención, adaptados al riesgo y necesidades del paciente, con especial atención a la transición. Finalmente, se comenta la situación actual de los SCI en España y consideramos las perspectivas futuras que contribuyan a mejorar la salud y calidad de vida de esta población.
Childhood cancer encompasses a heterogeneous group of malignant neoplasms for which overall survival has increased markedly in recent decades, currently exceeding 80%–85% in most developed countries.1–4 Advances in diagnostic methods, surgical techniques, intensive chemotherapy and targeted therapy, radiation therapy modalities, supportive care, knowledge of tumour biology, multidisciplinary management and the creation of large multicentre and international collaborative groups are, combined, key contributors to the increase in survival. Participation in clinical trials is currently considered the standard of care.3
However, as D’Angio stated in 1975,5 “cure is not enough”. The improvement in survival has led to a considerable increase in the number of childhood cancer survivors (CCSs) that reach adulthood.4 Decades of research have shown that childhood cancer cure is associated with, in comparison to the general population, and increased risk of early mortality, the development of second cancers and a broad range of late clinical and psychosocial effects resulting from the disease or the received treatment.3,6–10 A growing awareness of late toxicity has led to the stratification of patients into risk groups for each type of cancer based on clinical, biological or genetic characteristics in order to modulate the intensity and toxicity of treatment, which has not resulted in a reduced cure rate but has achieved a reduction in mortality due to late effects in CCSs who were treated more recently,10,11 and has also prompted changes in the risk models for certain chronic conditions.2 Lifelong follow-up is considered essential and is indicated in every CCS for the prevention, early detection and management of these problems and delivery of interventions to prevent or improve their adverse outcomes.12
At present, the scientific output in a broad range of fields related to CCSs is increasing at a quick pace, which calls for summarising scientific findings to yield high-quality evidence that can significantly improve the management of cancer survivors. Nevertheless, significant challenges still remain that we want to address in this article, such as determining the actual magnitude of morbidity and mortality in long-term CCSs, the impact of factors that determine the individual risk for late effects, the importance of harmonising long-term follow-up guidelines and the challenges encountered in implementing them. Lastly, we will discuss the current situation in Spain as regards the follow-up of CCSs and future perspectives for this population.
Morbidity and mortality in long-term childhood cancer survivorsThe most common late effects of paediatric cancer encompass several broad domains, including growth and development, organ and system function, second cancers, early mortality and psychosocial problems.3,8,10,13 This has an impact on physical, cognitive and emotional health and on the adequate performance of the patient’s role at home, in school, at work and in the community,14 with an impact on health-related quality of life. Overall, it is estimated that 60%–90% of CCSs develop one or more chronic health problems and 20%–80% experience severe or life-threatening complications.6,15 The late effects described in CCSs are traditionally classified into:
- a)
Early mortality: childhood cancer survivors are at increased risk compared to the general population,9,10 chiefly on account of second cancers, complications of late effects and premature aging described in cancer survivors.16,17
- b)
Physical problems: excellent reviews in the literature have analysed neurologic and neurocognitive,10,13,18 endocrine-metabolic,10,19 cardiovascular,10,20,21 gastrointestinal,10,22 respiratory,10,21 musculoskeletal,10,23, renal,10,22 reproductive10,24 and sensory10,25 problems as well as second tumours.10,26 We will not undertake a detailed description of these burdens due to the limited length of the article, so we provide the reader with the pertinent references.
- c)
Psychosocial problems: they are often combined and develop more frequently in survivors with a greater burden of organic disease. Thus, although 80%–90% of CCSs report that they feel fine,13 mental health and psychiatric problems are more prevalent in them compared to their siblings and the general population.10,13 The described problems include emotional distress,13 anxiety,27 depression,28,29 suicidal ideation,28 somatization and post-traumatic stress.13,27 Other exhibit positive psychological features of post-traumatic growth and resilience.27 In the social sphere, either due to the presence of neurocognitive deficits, other severe late effects or the experience of cancer in itself, survivors may experience difficulties in their relationship with their peers, school failure, less frequent access to university, which translates to career problems and unemployment, and, in consequence, decreased independence from the family, decreased economic resources, difficulties in their relationships with partners or spouses, less frequent marriage and fewer offspring.13,27,28
Considering the heterogeneity of the associated health problems, estimating the actual magnitude of the morbidity and mortality in CCSs poses a veritable challenge to clinicians and researchers. For decades, a large amount of evidence has been produced on the subject, and the variability observed in the data is due to a substantial methodological heterogeneity in terms of the population under study (age, sample size, representativeness, type of cancer, hospital-based vs population-based, duration of follow-up), the study design (cross-sectional, case-control, cohort, clinical trial, systematic review), the source of the data (population registers, patient self-reports, clinical assessments), the outcomes under study (mortality, adverse event) and how those outcomes are measured (survival, prevalence, cumulative incidence, risk), with limitations due to selection and information bias that have an impact on the reliability of the results and the comparability of studies.
Similarly, the broad range of reported effects requires a classification and grading system and the use of uniform terminology in their reporting. In 2016, the National Cancer Institute 2016 developed its Common Terminology Criteria for Adverse Events (CTCAE), establishing 168 specific chronic problems and 5 severity categories: grade 1 (mild), grade 2 (moderate) grade 3 (severe/disabling), grade 4 (life-threatening) and grade 5 (death). Since this rubric was not fully representative of the adverse effects on growth and physical and cognitive development in CCSs, Hudson et al.30 adapted the classification and severity grading of long-term adverse outcomes to make it applicable to CCSs, modifying some of the current criteria CTCAE version 4.03 criteria and introducing others that had not been included or addressed optimally, giving rise to a total of 208 late medical or neuropsychiatric adverse events, excluding second cancers.
On the other hand, traditional measures, like the prevalence or cumulative incidence, frequently used to estimate the magnitude of events, only involve simple counts or proportions observed until the first event,7,14 resulting in underestimation. The real price of survivorship is reflected in the cumulative burden of disease or morbidity experienced, taking into account the development and severity of multiple medical problems and recurrent events in the individual patient, something referred to as “multimorbidity”.31 Depending on given determinants (risk factors, type of tumour, lifestyle factors…), multimorbidity fits certain patterns (clustering of adverse events in different combinations) that may change over time. Furthermore, the development of a given event could help predict the development of the next event.31 Thus, the use of new endpoints, such as the mean cumulative count or the cumulative burden (number of events, independently of the type), allows a more precise assessment of multimorbidity by describing both the severity and the diversity of adverse health effects. In addition, these approaches, contrary to traditional measures, account for events that constitute a competing risk (event that precludes the occurrence of the event of interest), offering more accurate results on the actual magnitude of the problem.7,14Fig. 1 shows how the distribution of the cumulative burden of grade 3–5 events in CCSs, significantly higher compared to controls in the community, generally increases with age and in relation to the type of cancer.7
Distribution of cumulative burden (grades 3-5) in St Jude Lifetime Cohort Study childhood cancer survivors and community controls by diagnosis group and age.
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CNS, central nervous system tumours; NHL, non-Hodgkin lymphoma; STS, soft tissue sarcoma. Bone tumour: osteosarcoma and Ewing sarcoma.
Source: Bhakta et al.7
Another important advance is the use of simulation models to determine the individual risk/ probability of certain events based on the characteristics of the survivor (age, exposure, follow-up, comorbidities) extrapolating data obtained outside the observation period, which has an impact on the assessment of the risk of mortality and life expectancy, the selection of screening strategies and follow-up recommendations.14
Furthermore, the undertaking of large cohort studies of CCSs in Europe and North America in the past 25 years has been essential to collect data on the burden of disease after treatment.8. Table 1 summarises the results of some of these studies, which we highlight on account of the sample size and their contribution to the knowledge of adverse events in CCSs.2,6,8,11,15,30–33
Large cohorts of CCSs and their contribution to the evidence on late effects.
| Cohort | Description | Contributions |
|---|---|---|
| Cancer Children Survivors Study (CCSS) | Cohort established in 1994 in the USA and Canada: 26 centres.Institution-based retrospective cohort with prospective follow-up.Cohort: 1970−1999.Total active CCSs: 25 664.Control cohort: siblings.Any type of cancer | • 370 publications• 2006: nearly three fourths of CCSs treated between 1970 and 1980 had at least one chronic condition, with severe conditions in more than 40%. The incidence of chronic conditions increased with the duration of follow-up.6• 2014: by age 50 years, the cumulative incidence of grade 3−5 events in CCSs was 53.6%, compared to 9.8% in controls.15• 2016: current regimens that reduce treatment exposure have increased the life expectancy in CCSs.11• 2018: the CI of grade 3−5 events decreased at 20 years from 33.2% (1970s) to 27.5% (1990s). The CI in siblings varied from 5.05% to 4.6%.2 |
| St Jude Lifetime Cohort Study (SJLIFE) | Established in 2007 in the USA.Retrospective institution-based cohort with prospective follow-up.Total active CCSs: 6000.Controls: community.Any type of cancer | 120 publications• 2017: adaptation of the common terminology criteria for adverse events (CTCAE 4.03) to the CCS population.30• 2017: by age 50 years, individual CCSs had experienced an average of 17.1 chronic health conditions of grade 1−5, of which 4.7 were grade 3−5, with a significantly greater burden compared to controls7 |
| British Childhood Cancer Survivor Study (BCCSS) | Established in 1998 in the UK.Population-based retrospective cohort:active CCSs: 18 000.Controls: national population.Any type of cancer | • 2017: risk stratification is necessary in CCSs to deliver evidence-based long-term follow-up care |
| Adult Life After Childhood Cancer in Scandinavia (ALiCCS) | Population-based retrospective cohort:Active cohort: 33 160 CCSs.Controls: population-based.Any type of cancer | • Assessment of the endocrine risk profile of CCSs treated in Northern Europe. At age 60 years, the cumulative risk in CCSs of having endocrine disorders requiring hospital contact was 40%32 |
| PanCare Network European consortium | Established in 2008: 12 EU countries.Retrospective and prospective population- and institution-based cohort.Total CCSs: 83 333 | Research projects:• PanCareSurFup (www.pancaresurfup.eu): focused on heart disease, second cancers and mortality in CCSs. Development of follow-up guidelines.• PanCareLIFE (www.pancarelife.eu): focused on female infertility, cisplatin ototoxicity and quality of life• PanCareFollowUp (www.pancarefollowup.eu): focused on the institution of state-of-the-art late effects clinics for surveillance of late effects according to international guidelines and delivery of a novel comprehensive care model for CCSs, including ICT tools |
CCS, childhood cancer survivor; CI, cumulative incidence; EU, European Union; ICT, information and communication technology; UK, United Kingdom; USA, United States of America.
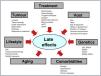
Although late effects have historically been associated with cancer and its treatment, several factors converge in the risk to experience them.10,16Fig. 2 presents the most thoroughly researched risk factors. Tables 2 and 3 summarise the risks associated with the use of radiotherapy and chemotherapy,10,34 including new targeted therapies that are more effective and less toxic and have improved survival in different childhood cancers.16 Notwithstanding, prolonged surveillance is recommended to determine whether emerging therapies are associated with improved long-term outcomes.16
Factors involved in the development of late effects in CCSs.
Treatment.Surgery: includes limb amputations and limb sparing surgery, cystectomy, oophorectomy or orchiectomy, lung resection, neurosurgical procedures, etc. Scars and deformities. Radiotherapy: the toxicity depends on the total dose, dose fraction, volume of the irradiated organ or tissue, type of radiotherapy technique (intensity-modulated RT and proton therapy are associated with a reduction in adverse events). The lower the age, the greater the damage.3Chemotherapy: depends on the agent, dose intensity, cumulative dose, schedule. Multimodal treatment: greater risk. Haematopoietic stem cell transplantation (HSCT) and graft-versus-host disease (GvHD): Both are associated with multiple adverse events.
Host. Age: < 1 year > 10 years, poorer prognosis, except in neuroblastoma10 (better prognosis with age < 1 year). Sex, race and ethnicity: modify risk in specific tumours.3Duration of follow-up: the longer the follow-up, the greater the risk. Each additional year constitutes an additional risk to develop adverse events.31Hormone environment and pubertal status: affect the susceptibility to chemotherapy and radiotherapy. Low levels of neuron-specific enolase associated with decreased survival.3
Tumour. all described factors have an impact on the risk of adverse events.
Genetics. Second tumours: associated gene variants14,26–27: BRCA (mama), P53 (Li Fraumeni). Cardiomyopathy: changes in RARG, genes involved in the transport of drugs and enzymes responsible for cardiotoxic metabolites.21Ototoxicity: changes in genes involved in the pharmacokinetics of cisplatin (ERCC).35Reproductive health: variants of BRSKI, associated with premature menopause. Single-nucleotide polymorphisms (SNPs) in androgen receptors in the testes are associated with oligospermia and azoospermia.14
Premature aging. Childhood cancer survivors exhibit accelerated aging that, in turn, is associated with the development of health problems. A study found higher rates of frailty in CCSs at a median age of 33 years than observed in the general population in the seventh decade of life.16
Comorbidities. Obesity, hypertension, metabolic syndrome, diabetes and chronic diseases increase the risk of late effects.
Lifestyle. intervention studies have demonstrated that physical activity is associated with a decreased morbidity in CCSs.31 Substance use (alcohol, tobacco, illicit drugs) is associated with an increased morbidity.44
Common chemotherapy-related late effects.
| Agent | Toxicity |
|---|---|
| Anthracyclines: doxorubicin, daunorubicin, epirubicin, mitoxantrone | Cardiovascular: LV systolic dysfunction/heart failure, ischaemic heart disease, pericardial disease, arrhythmias, valvular heart disease, peripheral vascular disease and stroke.Second cancers: breast cancer, sarcomas |
| Alkylating agents: cyclophosphamide, ifosfamide, procarbazine, temozolomide, dacarbazine, busulfan | Reproductive: oligospermia or azoospermia in male patients, abnormal ovarian function/diminished ovarian reserve, premature ovarian failure, Leydig cell dysfunction (procarbazine, temozolomide, dacarbazine, busulfan)Renal/vesical: glomerular and tubular injury, Fanconi syndrome, hypophosphataemic rickets, haemorrhagic cystitis (cyclophosphamide and ifosfamide)Second cancers: cyclophosphamide (bone and soft tissues, acute myeloid leukaemia, breast cancer), procarbazine (gastrointestinal), dacarbazine and busulfan (myelodysplastic syndrome and myeloid leukaemia)Cardiovascular: stroke, LV systolic dysfunction/heart failure, pericardial disease, arrhythmiaRespiratory: subclinical pulmonary dysfunction, interstitial lung disease, pulmonary fibrosis, restrictive lung disease, obstructive lung disease (busulfan)Ocular: cataracts (busulfan)Endocrine: primary hypothyroidism (busulfan) |
| Metal compounds: cisplatin, carboplatin | Neurologic: sensory peripheral neuropathyHearing: sensorineural and conductive hearing loss, tinnitus, vertigoRenal/genitourinary: glomerular and tubular injury, Fanconi syndrome, hypophosphataemic rickets. haemorrhagic cystitis, fibrosis vesical, vesicoureteral reflux, hydronephrosis, voiding dysfunctionReproductive: premature ovarian failure, spermatogenic failure in male patients (oligospermia and azoospermia)Cardiovascular: stroke, arrhythmias, vascular disease and myocardial ischaemia |
| Alkaloids: vincristine, vinblastine | Neurologic: sensory or motor peripheral neuropathyCardiac: myocardial ischaemia |
| Antimetabolites: methotrexate, cytarabine | Neurologic: leukoencephalopathy, headaches, seizures, sensory deficits, neurocognitive deficits (executive functioning, memory, attention, processing speed), learning deficit, low IQ, behavioural changesHepatic: dysfunctionRenal: kidney failure, hypertensionBone: low bone density (methotrexate), osteonecrosisCardiac: pericardial disease, arrhythmias, myocardial ischaemia and heart failure (cytarabine) |
| Mercaptopurine, tioguanine | Hepatic: hepatic impairment, fibrosis secondary to hepatic veno-occlusive disease |
| Corticoids: prednisone, dexamethasone | Bone: low bone density, necrosis avascular, growthOcular: cataractsMetabolic: obesity, metabolic syndrome |
| Nitrosoureas: carmustine, lomustine | Respiratory: subclinical pulmonary dysfunction, interstitial lung disease, pulmonary fibrosis, restrictive lung disease, obstructive lung diseaseReproductive: premature ovarian failure, spermatogenic failure in male patients, Leydig cell dysfunctionSecond cancers: myelodysplastic syndrome and acute myeloid leukaemia |
| Bleomycin | Respiratory: subclinical pulmonary dysfunction, interstitial lung disease, pulmonary fibrosis, restrictive lung disease, obstructive lung disease, respiratory distress syndrome |
| Topoisomerase inhibitors: etoposide, teniposide | Second cancers: myelodysplastic syndrome and acute myeloid leukaemia |
| Tyrosine-kinase inhibitors: imatinib, sorafenib, sunitinib | Endocrine: growth, primary hypothyroidismCardiovascular: LV systolic dysfunction/heart failure, arrhythmias, ischaemia, stroke, peripheral vascular disease |
| Immune checkpoint inhibitors o: nivolumab, ipilimumab | Endocrine: autoimmune hypophysitis with anterior pituitary gland dysfunction, autoimmune thyroid disease, growthCardiac: myocarditis and heart failure |
| VEGF inhibitors: bevacizumab | Cardiovascular: vascular disease, myocardial ischaemia, stroke, cardiomyopathy/heart failure |
| Proteasome inhibitors: bortezomib | Cardiac: heart failure, arrhythmias and myocardial ischaemia |
Anthracycline-induced cardiotoxicity depends on the cumulative dose. The risk increases with a cumulative dose > 250 mg/m2. Mitoxantrone: four-fold risk compared to doxorubicin. Daunoblastin and epiadriamycin: similar risk to that of doxorubicin. Cyclophosphamide-induced testicular toxicity depends on the cumulative dose (> 7.5 g/m2) and causes spermatogenic failure (oligospermia or azoospermia). Ifosfamide toxicity: cumulative dose > 60 g/m2. Lower risk in prepubertal patients. Cisplatin-induced ototoxicity depends on the cumulative dose of cisplatin (> 400 mg/m2). Lower risk with longer infusion times or fractionated schedules. Dental problems: any form of chemotherapy or irradiation administered to the oral cavity. In hematopoietic stem cell transplant recipients: toxicity associated with conditioning regimens: renal, hepatic, cutaneous, infectious, growth, endocrine, cardiovascular and respiratory problems and second cancers.
Common radiotherapy-related late effects.
| Organ, system | Irradiation field | Toxicity |
|---|---|---|
| Cardiovascular | Heart> 30 Gy> 20 Gy + anthracyclines> 10 Gy + large irradiation fieldNeck > 40 GyCranialTotal body irradiation | Arrhythmia, cardiomyopathy, heart failure, pericardial disease, valvular heart disease, ischaemic heart disease, conduction disorderCarotid or subclavian artery diseaseCerebrovascular disease: cavernomas, moyamoya, strokeDyslipidaemia |
| CNS | Cranial | Neurocognitive deficits (executive functioning, memory, attention, processing speed), learning deficit, low IQ, behavioural changesLeukoencephalopathy, cerebrovascular disease (stroke, moyamoya, occlusive vasculopathy) |
| Gastrointestinal | Any dose in oral cavity | Dental abnormalities, xerostomia, caries, osteonecrosis (> 40 Gy) |
| Oesophagus | Dysphagia and reflux | |
| Intestine (> 40 Gy) | Chronic enteritis, fistulas, stenosis | |
| Pelvis | Faecal incontinence | |
| Liver (> 30−35 Gy) | Liver dysfunction/failure, fibrosis, cirrhosis, cholelithiasis, FND | |
| Pancreas (> 10 Gy) | Diabetes mellitus | |
| Endocrine, metabolic and skeletal | Total body irradiation | Obesity, short stature, primary hypothyroidism, hyperthyroidism, thyroid nodules, thyroid cancer, GH deficiency, low bone density, glucose metabolism disorders, metabolic syndrome |
| Espinal | Disproportionate growth, scoliosis | |
| Cranial | Anterior pituitary hormone deficiencies, obesity, metabolic syndrome | |
| Facial | Facial bone and tooth abnormalities, osteoradionecrosis, poor cosmetic outcome | |
| Neck, thorax and mediastinum | Primary hypothyroidism, hyperthyroidism, thyroid nodules, thyroid cancer | |
| Abdomen, pelvis, genitourinary system | Glucose metabolism disorders, metabolic syndrome | |
| Long bones | Bone asymmetry, hypoplasia, fractures | |
| Immune system | Abdomen-spleen | Asplenia, hyposplenism |
| Reproductive system | Total body irradiation | Premature ovarian failure, spermatogenic failure in male patients |
| Cranial (hypothalamic-pituitary axis) or gonads, pelvis, abdomen (female patients) | Premature ovarian failure, spermatogenic failure in male patients and Leydig cell abnormalities | |
| Respiratory system | Chest | Obliterative bronchiolitis, interstitial pneumonitis, pulmonary fibrosis |
| Hearing | Cranial (occipital, posterior fossa) | Sensorineural and conductive hearing loss, vertigo |
| Vision | Cranial (visual pathways)Eye | Orbital hypoplasia, tear duct obstruction, xerophthalmia, keratitis, telangiectasias, impaired vision, retinopathy, optic neuropathy, enophthalmos, chronic eye pain, maculopathy, papillopathy, glaucoma |
| Urinary system | Kidney | Glomerular damage, proteinuria, hypertension |
| Second cancer | CranialNeckChestAbdomenExposed surfaceRadiotherapy in general | Meningiomas and gliomasThyroid cancerBreast cancerColorectal cancerNonmelanoma skin cancerRisk of leukaemia |
FND, focal nodular hyperplasia.
Anterior pituitary hormone deficiencies include: growth hormone (GH), follicle stimulating hormone/luteinizing hormone (FSH/LH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), prolactin, central precocious puberty.
The effects of surgery and chemotherapy can interact with the effects of cranial irradiation on an additive scale.
The role of genetics in the development of adverse events has attracted attention due to the variability in risk observed among individuals with similar exposure histories.14 Studies have chiefly focused on cardiomyopathy associated with treatment with anthracycline,21 cisplatin ototoxicity,35 reproductive health issues,14 neuropsychological impairment14 and second cancers.14,26,27 The possibility of carrying out genome-wide association studies (GWAS) has allowed the study of single-nucleotide polymorphisms, changes in genes associated with certain cancers, involved in DNA repair pathways or in the pharmacokinetics of chemotherapy agents. This is a new challenge for the near future that will yield valid data that will inform decision-making regarding first-line treatments and risk stratification during follow-up. Further research is needed to understand whether the joint effect of genetic susceptibility and treatment-related risks is additive or multiplicative and whether the pathophysiology underlying late effects in CCSs may differ from those seen in the general population.16
chemotherapy-induced and radiation-induced damage to normal, nonmalignant cells may explain the premature aging described in CCSs, and it may play a role worth investigating in the development of late effects.16 The molecular mechanisms associated with physiological aging in the general population may be exacerbated in CCSs (cellular senescence, telomere attrition, changes in DNA methylation patterns, accumulation of somatic DNA mutations, and mitochondrial dysfunction).16,17 This can result in a state of “frailty”, characterised by a decreased physiological reserve and impairment in organs and system, resulting in an increased vulnerability to stress, poorer physical performance, fatigue and increased risk of early mortality in CCSs compared to the general population.17
The challenge currently at hand is to be able to predict the individual risk to develop adverse events based on the combination of predisposing factors.
Importance of harmonising guidelines for long-term follow-upGuidelines for the long-term follow-up of CCSs have been developed to address the need to summarise the most relevant information, based on the available evidence of highest quality, regarding the health risks related to cancer and cancer treatment and to guide both survivors and health care providers on how to initiate proactive surveillance and implement interventions to optimise quality of life and life expectancy.12,36 Current and future challenges after guidelines are developed include their dissemination and implementation, the evaluation of their impact on quality of care quality survivor health outcomes and their periodic revision.35 Clinical practice guidelines (GPCs) can help deliver efficient and consistent care, avoiding variability, reducing costs and taking into account the views of patients. Thus, in the last two decades, several groups in North America and Europe have developed CPGs for the follow-up of CCSs37–40 with recommendations based on risk,32 although they differ on certain aspects. North American guidelines tend to approach late effects in relation to treatment exposure and European guidelines in relation to the affected organ or system. To reduce this heterogeneity, a worldwide collaboration was initiated in 2010 to harmonize guidelines for the long-term follow-up childhood and young adult cancer survivors and surveillance of late effects, giving rise to the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG: www.ighg.org),41 which provides recommendations while addressing which patients require surveillance, the appropriate surveillance modality, the time to initiate surveillance, the frequency of surveillance and the interventions available when problems are detected, taking into account differences in health care organization and available resources. The IGHG is a multidisciplinary group of experts on late effects (in the fields of paediatric and radiation oncology, medical and paediatric subspecialities, primary care, nursing and patient advocacy) in addition to experts in the development of CPGs. The current surveillance guidelines can be found at the IGHG website and are summarised in Table 4.
Available surveillance recommendations from the International Guideline Harmonization Group (IGHG, www.ighg.org).
| Specific area | Year of publication |
|---|---|
| Bone mineral density surveillance recommendations | 2021 |
| Breast cancer surveillance recommendations | 2020 |
| Cardiomyopathy surveillance recommendations | 2023 |
| Central nervous system neoplasm surveillance recommendations | 2021 |
| Coronary artery disease surveillance recommendations | 2021 |
| COVID-19 statement | 2020 |
| Dexrazoxane cardioprotection | 2022 |
| Fatigue surveillance recommendations | 2020 |
| Fertility preservation recommendations for female CAYA cancer patients | 2021 |
| Fertility preservation recommendations for male CAYA cancer patients | 2021 |
| Hepatic toxicity surveillance recommendations | 2021 |
| Hypothalamic-pituitary dysfunction surveillance recommendations | 2021 |
| Male gonadotoxicity surveillance recommendations | 2017 |
| Mental health problems surveillance recommendations | 2022 |
| Obstetric care recommendations: Counseling and surveillance in pregnancy | 2020 |
| Ototoxicity surveillance recommendations | 2019 |
| Premature ovarian insufficiency surveillance recommendations | 2016 |
| Psychosocial problems surveillance recommendations | 2022 |
| Recommendations for ongoing communication and ethical considerations for fertility preservation for patients with CAYA cancer | 2021 |
| Thyroid cancer surveillance recommendations | 2018 |
CAYA, childhood, adolescent and young adult.
In North America, the members of the Children’s Oncology Group GPG (COG-LTFU version 5.0)37 are strongly committed to the mission of the IGHG. They offer recommendations for CCSs based on risk, provide patient education materials and have developed the Passport for Care, a document for the individualised follow-up of patients. In Europe, the leadership in the follow-up of CCSs is the PanCare network (www.pancare.eu),42 which is carrying out several research projects funded by the European Union: PanCareSurFup (www.pancaresurfup.eu), on the development of IGHG recommendations and the transition to adult care, PanCareLIFE (www.pancarelife.eu), focused on female infertility, cisplatin ototoxicity and quality of life, and PanCareFollowUp (www.pancarefollowup.eu), on the development of IGHG guidelines and the design of the Sur-Pass or survivor passport, a form to summarise the treatment and record personalised recommendations.
The periodic evaluation of the impact of CPGs is an approach that contributes to guaranteeing the delivery of adequate care through the measurement of quality indicators (which are yet to be sufficiently developed for CCSs, an aspect that poses yet another challenge in the follow-up of these patients), with an emphasis on patient-centred care, taking account the patient’s preferences, satisfaction and values, which also demands a commitment by patients in taking responsibility for their self-care.36
Problems in the implementation of follow-up: models of careGiven the growing number of survivors, one of the main current challenges in their care is the implementation of long-term follow-up, implementing the recommendations of evidence-based CPGs in real-world clinical practice. The magnitude of this task does not only pose a challenge for adult or paediatric oncologists, primary care physicians, nursing staff, non-health care staff, caregivers and patients, whose coordination is essential, but also requires an infrastructure and organization that can allow the delivery of the personalised care and address the individual needs of survivors while also being based on the scientific evidence. The reality is that many CCSs do not receive adequate care.43
In the last 25 years, alternative care models have emerged that differ from the traditional model led by cancer specialists that is still predominant,43 supported by a multidisciplinary team with medical doctors from other subspecialities, psychologists and social workers. Table 5 summarises the characteristics, advantages and disadvantages of the proposed models. Tonorezos et al.44 analysed models of care for CCSs in 18 countries in all five continents. In general, with the exception of low-income countries, CCSs remain in follow-up through age 18 years (paediatric age) and for at least 10 years from diagnosis, and follow-up care is provided in paediatric care settings. An adequate transition to adult care for young adult CCSs and the delivery of individualised care are among the priorities in most countries. On the other hand, the training on late effects of health care professionals and patients themselves is considered essential. The health care authorities should establish the necessary infrastructure within the different health care systems to enable the delivery of the follow-up care that CCSs deserve.44
Models of care for childhood and adolescent cancer survivors.
| Type of Model | Description | Advantages | Drawbacks |
|---|---|---|---|
| Traditional: hospital-based | Implemented in survivor follow-up units located in the same hospital where the treatment took place by an experienced late effects care team.Most children’s hospitals deliver follow-up through age 18 years or at least 10 years from diagnosis | Experienced multidisciplinary care team (oncologists, surgeons, radiation therapists, nurses) supported by providers in other subspecialities, psychologists and social workers.Comprehensive care (social and psychological support).Close relationship between the patient and already known health care staff | Cannot take on all the patients due to infrastructure and resource constraints (costs).It is not the best follow-up option in CCSs at low risk of late adverse events.The distance from the home can be a disadvantage.In general, communication with PC is inadequate |
| Primary care | Care delivered predominantly or exclusively in primary care (PC) centres.The responsibility of follow-up lies with the general practitioner | Previous relationship with the provider.Greater access.More affordable care.Safe and effective alternative shown to be non-inferior in terms of outcomes and quality of life.Adequate patient satisfaction.Efficacious in patients with low risk of late effects.Cost-effective alternative for some types of adult cancer | Insufficient information about the cancer history.Lack of trust by oncologists in general practitioners.Requires training in follow-up of oncological patients and late effects.Lack of communication or coordination with hospital when hospital-based care is needed. |
| Shared care: oncology and primary care providers | Formal collaboration between cancer specialists and general practitioners to provide follow-up. The goal is to provide optimal cancer-specific care combined with optimal generalist care | Patients do not have to renounce to follow-up by cancer specialists.General practitioners feel safer.Patient satisfaction is high.Lower costs compared to traditional model.Solid communication between professionals, clearly defined roles and responsibilities. | May not be appropriate for patients at low risk of late effects.Requires specific training.Requires information on the previous cancer history.Lack of studies evaluating the surveillance of symptoms, comorbidities or the delivery of care to improve lifestyle habits |
| Long-term follow-up clinics | Specialised care out of the treating hospital.Multidisciplinary care (medical specialities, nursing and other health care professionals).May be offered within a shared care model in collaboration with PC | Adequate follow-up of complex patients at high risk of ate effects (adult CCSs, allogeneic bone marrow transplant recipients.High patient satisfaction | Requires substantial human and infrastructure resources.There are few data comparing this approach with the traditional model of care.Scarce availability |
| Care by oncology nurses | Care delivered by nurses specialised in cancer (in person, by telephone or online).Nurses offer education on healthy lifestyle habits and promote patient self-management | Professionals with training and experience in symptom control.Assist self-management.Provide guidance.Reduced follow-up costs.High patient satisfactionMediation between survivor and oncologist | Scarce availability.Requires substantial training.Scarce implementation to date |
| Supported self-management | The CCSs knows about late effects and manages his/her own care.Usually combined with other care models and with motivational interviewing | Good model for low-risk CCSs.Communication with caregivers.Improvement of quality of life, fatigue, sleep, anxiety, stress | Requires support and guidance, the development of healthy habits and having self-management skills |
The choice of model depends on patient-related factors, the characteristics of the health care system and national policy on follow-up. The preferences of survivors should always be taken into account. Some of the possible complements to the models of care include: comprehensive multidisciplinary rehabilitation care to address the physical, psychological, occupational and social problems that may emerge in complex patients; health care interventions promoting physical activity or the use of mobile applications (mHealth), allowing simpler communication in real time between survivors and providers, or telehealth models led by nurses with specific training.43 One example of a behavioural intervention is Oncokompass, an eHealth application to support cancer survivors based on self-management of symptoms by the survivor (Cancer Center Amsterdam).
All survivors should de provided with a personalised care plan (Passport of care or Sur-Pass) including a summary of the diagnosis and treatment received, any complications and late effects, the recommended follow-up based on risk, healthy lifestyle recommendations and support links, resources and selected educational materials. This document must be made available to the provider responsible for the follow-up during the care transition.
Long-term follow-up of CCSs in SpainIn Spain, the 2022 report of the Spanish Register of Childhood Tumours of the Sociedad Española de Hematología y Oncología Pediátrica (SEHOP, Spanish Society of Paediatric Haematology and Oncology), which offers incidence data on paediatric tumours with a coverage of 95% of the population, included 32 266 cases in children aged 0–14 years with an overall 5-year survival in the 2014–2016 cohort of 82% (95% CI, 80.1 %–83.7 %). Of this total, 26 006 (1980–2016) are CCSs.4 The SEHOP has a working group on adverse events and second cancers that published recommendations for follow-up based on international guidelines in 2012.45 Spain has participated in meetings of the PanCare consortium, which allowed access to the Survivorship Passport translated to Spanish, sharing experiences with different working groups and learning about the infrastructure and multidisciplinary staff required to manage follow-up in many areas of health. Despite a substantial interest on the subject, the overall magnitude of these problems has yet to be established in Spain, and it has not been possible to implement follow-up homogeneously throughout the country, although an increasing number of centres is launching specialised clinics for long-term follow-up staffed by professionals with expertise and knowledge on the subject.
The future of childhood cancer survivorsThe management of CCSs is highly complex, and despite all the efforts invested in the development of guidelines and recommendations for worldwide implementation, the current evidence shows that care continues to be suboptimal, especially in low-income countries.12
The identified challenges evince the need to increase our knowledge on the late effects and psychosocial impact of childhood cancer, including the toxicity of novel therapies, the contribution of genetic factors to risk, multimorbidity, morbidity clustering patterns and the prediction of individual risk. International collaboration in large cohort studies must be promoted to allow the development of treatment and follow-up strategies adapted to specific phenotypes in CCSs and interventions personalised according to their deficits. This, combined with the use of new technologies, signal the dawn of a new precision survivorship era.
The guideline harmonisation initiative must be promoted and maintained to ensure a standardised and consistent long-term follow-up, developing new recommendations based on risk stratification and scientific evidence and evaluating the impact of current guidelines.
The delivery of effective, feasible, high-quality and risk-appropriate follow-up care poses the greatest challenge, a nearly insurmountable one. It must be pursued by health care professionals supported by health care systems and health care authorities and policymakers, with allocation of the necessary resources to guarantee universal access to survivorship care. A care transition system is of the essence, which also requires coordinated efforts to overcome existing barriers and the creation of patient-centred survivorship care teams in addition to increasing the involvement and responsibility of patients in their own management. All survivors must have a personalised follow-up care plan in order to improve their future health.
Continuous and coordinated efforts are required from researchers, medical professionals and policymakers to address the needs of survivors and guarantee lifelong personalised follow-up for early detection of health care problems and psychosocial support. The general goal is to improve health and quality of life so that today’s increase in the number of children treated successfully does not translate into an increase in chronically ill adults tomorrow.5
FundingThis research project did not receive specific financial support from funding agencies in the public, private or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.